| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 1, Number 2-3, June 2012, pages 65-69
Diagnostic and Therapeutic Difficulties in Diffuse Large B-cell Lymphoma Arising From HHV8 Positive Castleman Disease
Edit Payera, d, Zsofia Miltenyia, Zsofia Simona, Ferenc Magyaria, Sandor Barnab, Gabor Mehesc, Arpad Illesa
aInstitute of Medicine, University of Debrecen, Medical and Health Science Center, H-4032 Debrecen, Moricz Zs. blvd. 22., Hungary
bScanomed Ltd., H-4032 Debrecen, Nagyerdei blvd. 98., Hungary
cInstitute of Pathology, University of Debrecen, Medical and Health Science Center, H-4032 Debrecen, Nagyerdei blvd. 98., Hungary
dCorresponding author: Edit Payer
Manuscript accepted for publication June 12, 2012
Short title: DLBCL Arising From Castleman Disease
doi: https://doi.org/10.4021/jh27w
| Abstract | ▴Top |
Diffuse large B-cell lymphoma arising from multicentric Castleman disease is an aggressive disorder with short survival. In 2001 our 46-year old patient presented cervical lymphadenopathy. The third bioptic sample proved HHV8 positive Castleman disease in 2002. Chemoterapy (steroid, cyclophosphamide, CVP, interferon-α, thalidomide) and radiotherapy was given with themporary success. In 2009 compression of the respiratory tract needed mantle irradiation, and rapid progression developed, inguinal biopsy revealed DLBCL. R-CHOP was started, but the interim PET/CT showed no respons, thus therapy was changed to R-DHAP, autologous stem cell transplantation was planned. The stem cell collection was made after 2 cycles of chemotherapy. However, further progression could be detected which seemed to be chemoresistant (R-IGEV, R-ICE). Palliative irradiation was performed but serious myelosuppression was developed which led to febrile neutropenia with pneumonia and sepsis. Despite of targeted antibiotic treatment the infection caused our patient’s death six months after the diagnosis of DLBCL. We would like to highlite the importance of the repeated biopsies if the behavior of a disease has changed and the rare disorders needed personalised treatment.
Keywords: Castleman disease; Diffuse large B-cell lymphoma; 18FDG-PET/CT
| Introduction | ▴Top |
Castleman disease (CD) is a rare, non-malignant lymph node hyperplasia [1], which was first described in 1954 [2]. Clinically it may adopt unicentric (localized) and multicentric presentation. Pathologically it is classified as hyaline vascular, plasmacytic or mixed cellularity types. The hyalin vascular variant is found in most unicentric cases, and the plasmacytic variant is found in most multicentric cases [3]. The pathogenesis of CD is not clearly understood, which is more frequent in human immundefitiency virus (HIV) infected patients [4]. Human herpes virus 8 (HHV8) and increased production of interleukin-6 (IL-6) can play an important role in the pathogenesis [3, 5]. Among inflammatory mediators IL-6 has been found to be especially relevant as it may act as a potent stimulus for the proliferation of B-cells and in experimental studies have been shown that it can provoke CD. Other possible mediators are vascular endothelial growth factor (VEGF) and interferon-α [6]. Clinical presetation is variable, ranging from asymptomatic disease to severe lymph node enlargement associated with systemic signs, often found in high grade lymphoma [3]. Unicentric type can be treated with excisional surgery or irradiation therapy, but in multicentric form immuno- and chemotherapy, furthermore antiviral therapy can be useful.
In the 2008 WHO lymphoma classification there is a new category known as large B-cell lymphoma associated with HHV8 positive mulicentric Castleman disease [7], which consists of monoclonal proliferation of HHV8 infected plasmoblasts. For the clinical manifestation lymph node and spleen enlargement is typical, but there can be disseminal disease also. It is an expressly aggresive disorder with a median survival of a few month [8].
With our case report we would like to highlight the difficulities of diagnosis, the importance of close follow-up and difficulties of treatment which necessitate developement of standardized protocols and exploration of new therapeutic agents.
| Case Report | ▴Top |
The 53-year old caucasean male presented with cervical lymph node enlargement in 2001, the histological analysis of a cervical lymph node showed reactive hyperplasia. No infection could be confirmed and systemic autoimmune disorder was ruled out. The laboratory findings showed decreased CD4/CD8 rate, increased T-cell activation, normal levels of erythrocyte sedimentation rate, lactate dehidrogenase (LDH) and beta-2 microglobulin. Because of lymph node enlargement in mediastinal, axillary, abdominal and inguinal regions at the end of 2001 and at the beginning of 2002 inguinal lymph node biopsy was repeated, which confirmed the previous diagnosis. Because the tonsills narrowing the upper respiratory truct oral steroid therapy was begun (32 mg/die methylprednisolone) which resulted in moderate regression. A third biopsy in June 2002 proved Castleman disease (Fig.1A, B, C) and HHV8 positivity was also detected. Steroid therapy (16 mg/die) was continued but side-effects appeared, thus we changed to cyclophosphamide monotherapy (firstly 1000 mg in infusion, after that 2 x 50 mg/week orally on one day). In October 2002 progression was noted in the cervical lymph nodes, so CVP (1400 mg cyclophosphamide, 2 mg vincristine, 125 mg metilprednisolon) chemotherapy and interferon-α-2b treatment (3 x 3 MU/week) was started which seemed to be effective. However in March 2003 enlargement of cervical lymph nodes and tonsills were detected and involved field radiation therapy (40 Gy absorted dose) was performed. Because of its effectivity the radiation of the ingunal and axillary region was followed, and regression was established in these areas. During the regular follow-up there were no significant events. From April 2007 thalidomide monotherapy (200 mg/die) was given. At that time a 18FDG-PET/CT scan was performed, which detected enlarged lymph nodes nearly in all lymphatic regions (Fig. 2A). However in February 2009 radiation therapy (40 Gy) was administered again because of respiratory problems caused by mediastinal lymphadenomegaly. A biopsy was performed in March 2009 from ingunal region, in which the growth of lymph nodes was the most significant, and the histological analysis established T-cell/histiocyte rich diffuse large B-cell lymphoma (Fig.1D, E, F). A staging 18FDG-PET/CT scan showed lymph node enlargement, especially in infradiaphragmatic regions (Fig. 2B). Iliac crest biopsy did not show bone marrow involvement by lymphoma. During physical examination lymph node enlargement in all peripherial regions, hepatosplenomegaly and in the bioptic wound an abscess were detected. The laboratory tests showed pancytopenia, significantly eleveted levels of LDH, C reactive protein and direct Coombs positivity. From the abscess Bacteroides fragilis and Staphylococcus aureus were cultured, which was treated with targeted intravenous antibiotics and necrectomy was performed daily. When inflammation decreased dose dens R-CHOP-14 (700 mg rituximab, 1500 mg cyclophosphamid, 2 mg vincristine, 100 mg adriamycin, 64 + 32 mg metilprednisolone, on day 1 to 5) therapy was started, but the interim 18FDG-PET/CT scan (Fig. 2C), made before the third cycle, detected moderate therapeutic response, so we changed to R-DHAP (700 mg rituximab, 150 mg cisplatine on day 1, 2 x 3500 mg cytosine arabinosid on day 2, 40 mg dexamethasone/die on day 1 to 4) and planned autologous haemopoetic stem cell transplatation. With physical examination the size of the peripherial lymph nodes and spleen decreased, thus stem cell collection (2.8 x 106/kg) was performed in June 2009. Due to the chemotherapies severe pancytopenia and infectious side-effects developed. Reduced dose of R-IGEV (700 mg rituximab, 30 mg vinorelbin on day 1, 3000 mg ifosfamide/die on day 1 to 4, 1200 - 1000 mg gemcitabine on day 1 and 4) and R-ICE (700 mg rituximab, 100 mg etopozide on day1 to 3, 5000 mg ifosfamide on day 2, 500 mg carboplatine on day 2) was given because of inadequate response. However both clinically and by 18FDG-PET/CT scan (Fig 2D) further significant progression was established in the abdominal region and in the same time our patient was in bad general condition (ECOG PS 4) and severe myelosuppression was present with serious bacterial infections. As further therapy was needed to control the disease vepeside-dexamethasone (100 mg vepeside on day 1, 40 mg dexamethason on day 1 - 4) therapy was given in reduced dose and extended field irradiation to the inradiaphragmatic regions and spleen (in 2-2 Gy fractions to each region) were performed. Colitis caused by the radiation therapy, Escherichia coli septicaemia and fungal infection developed which could not be controlled and caused the patient’s death. The autopsy confirmed the presence of DLBCL in the lymph nodes, bone marrow, liver and spleen, and the direct cause of death was heart failure and pneumonia.
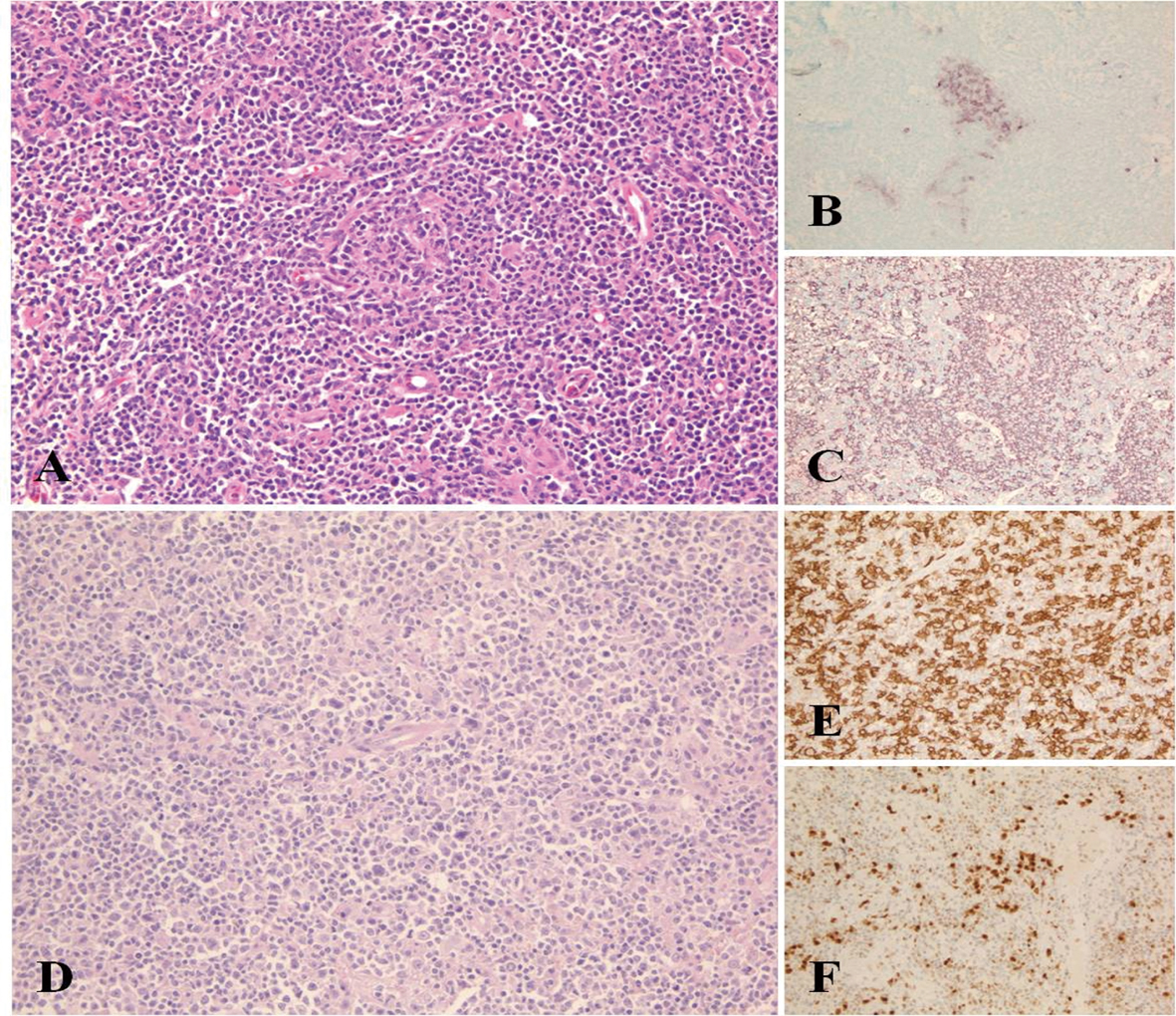 Click for large image | Figure 1. Morphological appearance of Castleman disease diagnosed at initial lymph node sampling. Compressed and partially hyalinized germinal centre surrounded by mantle zone B-cells HE, (A). Residual GC are highlighted by CD10 immunostaining of the same region (B) and remain negative by CD20 due to involution and hialinization (C). Diffuse large B-cell lymphoma showing solid areas of large CD20+ cells (D, E). MUM1 positivity supports the activated B-cell origin of the change (F). |
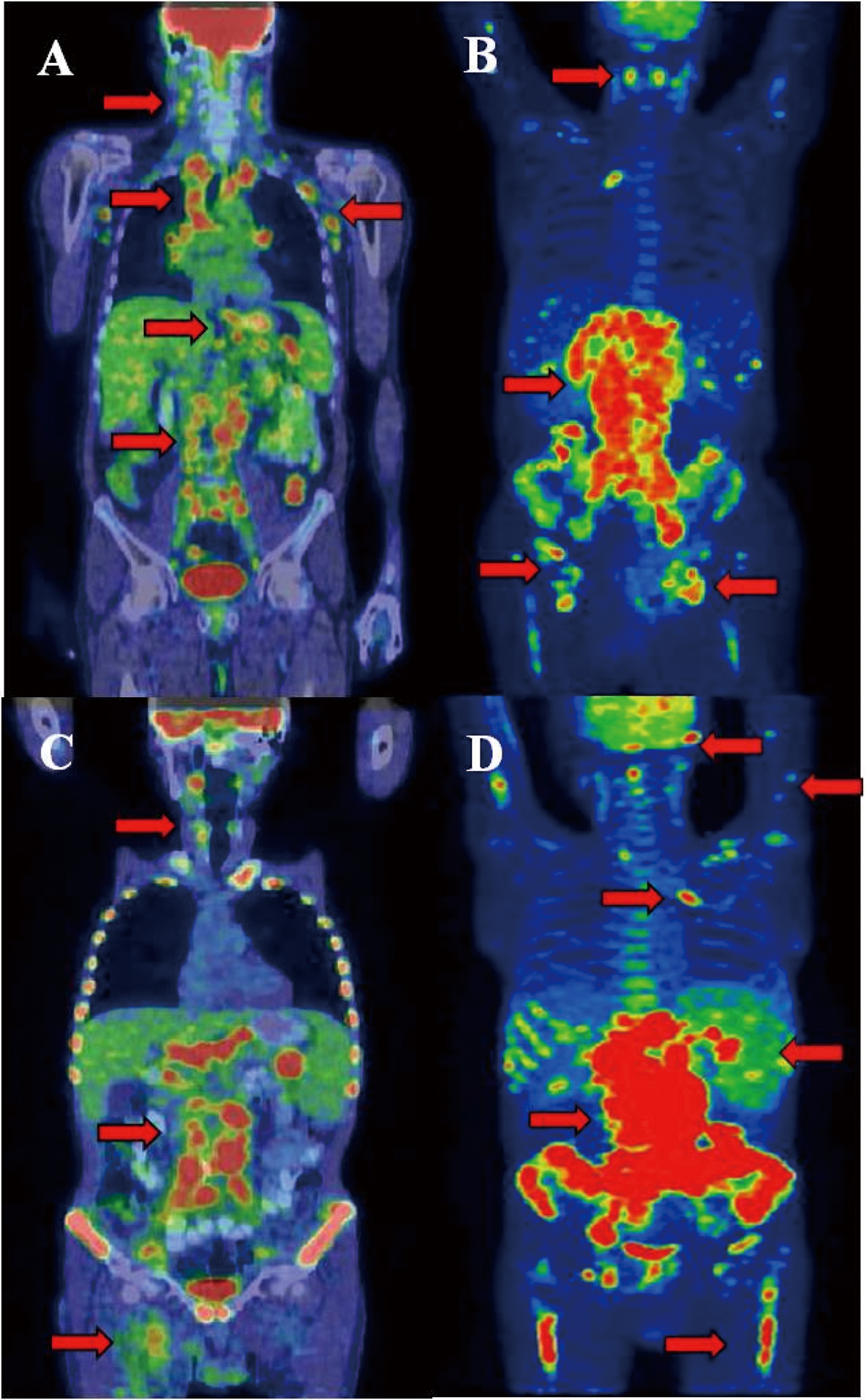 Click for large image | Figure 2. Castleman disease showed activity in many lymphatic areas (A). During staging of DLBCL significant progression was detected in the infradiagphragmatic region with bone marrow involvement (B). After 2 cycles of R-CHOP interim PET/CT proved improvement in the supradiaphragmatic, but not in the infradiaphragmatic region. After several chemotherapeutic protocols refractory state was confirmed by PET/CT and clinical signs (D). Arrows show the involved lymph nodes, spleen, lung and bone marrow. Arrows shows tracer enhanced areas. |
| Discussion | ▴Top |
Our pateint’s illness had more diagnostic and therapeutic difficulties. Firstly, four biopsies were needed to proved the diagnosis of multicentric Castleman disease (MCD). On the other hand after diagnosis of MCD we tried severel chemotherapeutic protocols without significant result, 7 years after diagnosis we detected the transformation into DLBCL and its treatment also presented terapeutic challenges. Most reports suggest that patients who develop lymphoma do not respond well to standard chemotherapy. The low number of cases do not allow to establish standardized protocols. For effective treatment the pathophysiology of the disease is needed to be explored, but nowadays it is still not clearly understood.
In reported data HHV8 has been shown to encode functional viral homologues of human proteins that play a role in cell signalling pathways, regulation of cell-cycle, obstruction of apoptosis, furthermore it is similar to genes of cytokins and cytokin receptors which are important to cell proliferation and transformation [9, 10]. HHV8 is known to encode a viral- IL-6 (vIL-6), with approximately 50% similarity to the human IL-6 at amino acid level [3]. IL-6 causes proliferation and differentiation of B-cells into antibody producing cells, resulting hyeprplastic follicules and lymph node enlargement. IL-6 also increases VEGF secretion, causing angiogenesis, proliferation of vascular muscle cells and capillary proliferation with endothelial hyperplasia. Moreover, VEGF can also initiate IL-6 secretion in the endothelial cells of the microcapillaries [11]. IL-6 is also responsible for polarization of T-lymphocytes to a type 2 cytokin profile leading to Ig production and autoimmun phenomena [12]. It can be hypothetized that Castleman disease and non-Hodgkin lymphoma result from dysregulated IL-6 production induced by HHV8 vIL-6, leading to B-cell malignancy by clonal outgrowth, in conjunction with other viral potential oncogenes, especially the viral homologue of cyclin D1, that control cell-cycle progression from G1 to S phase [13-15]. It has been reported that patients with MCD often develop secondary tumors, such as Kaposi sarcoma, non-Hodgkin lymphoma, Hodgkin disease and plasmacytoma [16, 17]. Prevalence of lymphoid malignacy in CD is difficult to establish because of the low number of the cases. Hodgkin disease occurs in localized CD of plasma cell type, usually in the same areas. Hodgkin disease is interfollicular or nodular sclerosis type and its clinical course seems to be better than NHL. Non-Hodgkin lymphoma has been reported in up to 18% of MCD [18]. Immunoblastic and plasmablastic B-cell lymphoma is the most frequent subtype [19, 20]. The developed NHL is tipically high grade type with stage III or IV at the time of diagnosis, extanodal organs are involved, and an aggressive clinical course can be seen with short survival [21]. In MCD HHV8 positive plasmablasts tipically occur as isolated cells but may form aggregates that show λ light-chain restriction and therefore have been referred to as microlymphomas. In some cases these plasmablasts may form frank plasmablastic lymphoma [22]. HHV8 may infect IgM-positive naive B-cells and drive these cells to differentiate into plasmablasts without undergoing the germinal center reaction, during which normal naive B-cells mutate into plasma or memory B-cells [23].
In the case of our patient the fourth biopsy showed the objective diagnosis of Castleman disease during one year. The treatment was partly successfull only. After 7 years it developed into DLBCL, which was refractory to the polychemotherapy (R-CHOP, R-DHAP, R-IGEV, R-ICE, Vepesid-Dexamethason) and radiotherapy. At least the progression of the lyphoma and infectious side-effects caused the patients death.
With our case report we suggest that patients with CD are needed close follow-up and if the behaviour of the disease has changed, biopsy should perform. Moreover, for the right diagnosis the cooperation between the different professions is also indispensable.
Acknowledgments
This work was supported by ETT 200/2009.
Conflict of Interest
All authors have no conflicts of interest.
| References | ▴Top |
- Frizzera G. The distinction of Hodgkin's disease from anaplastic large cell lymphoma. Semin Diagn Pathol. 1992;9(4):291-296.
pubmed - Castleman B, Towne VW. Case records of the Massachusetts General Hospital; weekly clinicopathological exercises; founded by Richard C. Cabot. N Engl J Med. 1954;251(10):396-400.
pubmed - Casper C. The aetiology and management of Castleman disease at 50 years: translating pathophysiology to patient care. Br J Haematol. 2005;129(1):3-17.
pubmed doi - Stebbing J, Pantanowitz L, Dayyani F, Sullivan RJ, Bower M, Dezube BJ. HIV-associated multicentric Castleman's disease. Am J Hematol. 2008;83(6):498-503.
pubmed doi - van den Berge M, Pauwels P, Jakimowicz JJ, Creemers GJ. Hyaline vascular Castleman's disease: a case report and brief review of the literature. Neth J Med. 2002;60(11):444-447.
pubmed - Dham A, Peterson BA. Castleman disease. Curr Opin Hematol. 2007;14(4):354-359.
pubmed doi - Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009:523-531.
pubmed doi - Isaacson PG, Campo E, Harris NL. Large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease. In: Swerdlow SH, Campo E, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. Geneva: WHO Press, 2008: 258-259.
- Ganem D. KSHV and Kaposi's sarcoma: the end of the beginning? Cell. 1997;91(2):157-160.
pubmed doi - Sharp TV, Boshoff C. Kaposi's sarcoma-associated herpesvirus: from cell biology to pathogenesis. IUBMB Life. 2000;49(2):97-104.
pubmed doi - Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, et al. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391(6662):86-89.
pubmed doi - Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, Nakahata T, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989;74(4):1360-1367.
pubmed - Chang Y, Moore PS, Talbot SJ, Boshoff CH, Zarkowska T, Godden K, Paterson H, et al. Cyclin encoded by KS herpesvirus. Nature. 1996;382(6590):410.
pubmed doi - Menke DM, Tiemann M, Camoriano JK, Chang SF, Madan A, Chow M, Habermann TM, et al. Diagnosis of Castleman's disease by identification of an immunophenotypically aberrant population of mantle zone B lymphocytes in paraffin-embedded lymph node biopsies. Am J Clin Pathol. 1996;105(3):268-276.
pubmed - Staskus KA, Sun R, Miller G, Racz P, Jaslowski A, Metroka C, Brett-Smith H, et al. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. J Virol. 1999;73(5):4181-4187.
pubmed - Oksenhendler E, Duarte M, Soulier J, Cacoub P, Welker Y, Cadranel J, Cazals-Hatem D, et al. Multicentric Castleman's disease in HIV infection: a clinical and pathological study of 20 patients. AIDS. 1996;10(1):61-67.
pubmed doi - Gould SJ, Diss T, Isaacson PG. Multicentric Castleman's disease in association with a solitary plasmacytoma: a case report. Histopathology. 1990;17(2):135-140.
pubmed doi - Peterson BA, Frizzera G. Multicentric Castleman's disease. Semin Oncol. 1993;20(6):636-647.
pubmed - Weisenburger DD, Nathwani BN, Winberg CD, Rappaport H. Multicentric angiofollicular lymph node hyperplasia: a clinicopathologic study of 16 cases. Hum Pathol. 1985;16(2):162-172.
pubmed doi - Hall PA, Donaghy M, Cotter FE, Stansfeld AG, Levison DA. An immunohistological and genotypic study of the plasma cell form of Castleman's disease. Histopathology. 1989;14(4):333-346; discussion 429-332.
pubmed - Frizzera G. Castleman's disease and related disorders. Semin Diagn Pathol. 1988;5(4):346-364.
pubmed - Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, Weiss RA, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95(4):1406-1412.
pubmed - Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111-126.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


