| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 5, Number 3, September 2016, pages 83-93
Splenomegaly in Patients With Sideropenic Anemias: Clinical and Hematologic Significance
Safaa A. A. Khaleda, c, Gehan S. Seifeldeinb
aDepartment of Internal Medicine, Hematology & BMT Unit, Assiut University Hospital, Faculty of Medicine, Assiut University, Assiut, Egypt
bDepartment of Diagnostic Radiology, Assiut University Hospital, Faculty of Medicine, Assiut University, Assiut, Egypt
cCorresponding Author: Safaa A. A. Khaled, Department of Internal Medicine, Hematology & BMT Unit, Assiut University Hospital, Assiut, Egypt
Manuscript accepted for publication August 25, 2016
Short title: Splenomegaly in Sideropenic Anemias
doi: http://dx.doi.org/10.14740/jh286w
| Abstract | ▴Top |
Background: Sideropenic anemias (SAs) are a group of hypoproliferative anemias characterized by hyposideremia. Although they run an insidiously started slowly progressive course, they are a pointer for an underlying serious disease. Fortunately, in most cases, management of SAs is available, effective and relatively inexpensive. Splenomegaly was reported in patients with SAs with variation in Hacckett’s grading and hematological profile. Etiopathogenesis of splenomegaly in SAs was mainly explained as related to the underlying pathologic process of anemia or as a component of the rarely occurring Paterson-Kelly syndrome. Apart from these, etiopathogenesis of splenomegaly in SAs is still a fruitful point for current research. The aim of the present study was to assess splenomegaly in patients with SAs in terms of frequency, clinical and hematological profile of splenomegaly in SAs. Another aim was to assess prognostic significance and assume etiopathogenesis of splenomegaly in SAs.
Methods: A prospective study was conducted on 83 patients with SAs and 25 normal sex- and age-matched healthy controls. Patients’ demographic, clinical and hematologic data were collected through thorough history and clinical examination. Splenomegaly was assessed with clinical examination of the study subjects, graded with Hachett’s clinical grading, and then confirmed with ultrasonographic examination. Patients were treated as per the published guidelines for treatment of SAs. Those with splenomegaly were subjected to a strict follow-up plan.
Results and conclusion: Analysis of the collected data showed that splenomegaly is of robust clinical and hematologic significance in patients with SAs.
Keywords: Sideropenic anemias; Splenomegaly; Clinical significance
| Introduction | ▴Top |
Sideropenic anemia (SA) is a hematologic term referred to anemia with reduced serum iron levels, including iron deficiency anemia (IDA), and anemia of chronic disease (ACD), or a dimorphic anemia of IDA and ACD. SAs are the most prevalent types of anemias worldwide, firstly IDA and secondly ACD [1-3].
In vitro and in vivo studies demonstrated reduced serum iron in patients with chronic inflammatory conditions, infections and malignancies. Inflammatory cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor-alpha are the main trigger for hypoferemia, and other bone morphogenetic proteins 2, 4, 6, and 9 produce the same effect in patients with malignancy. These effects were mediated through hepcidin [4-6].
The differentiation between IDA and ACD is quiet difficult, nevertheless in ACD, there is a confounding evidence of chronic infectious, inflammatory, or malignant disease causing the anemia. Furthermore, in ACD, the red blood cell (RBC) indices are usually normal (mean corpuscular volume (MCV) 80 - 100 fL, mean corpuscular hemoglobin concentration (MCHC) 32 - 36 g/dL and red cell distribution width (RDW) 12.0-14.6%), while in IDA, all RBC indices are below normal except the RDW which is commonly raised. Total iron binding capacity (TIBC) was found raised in IDA and reduced in ACD; however, serum hepcidin levels were considered the most important difference between IDA and ACD. Unfortunately, laboratory assay of serum hepcidin is difficult, expensive and not widely available. Soluble transferrin receptor (sTfR) was found to be a good differential test between IDA and ACD; it was found raised in patients with IDA; however, standardization of the test was difficult [7-9].
Hepcidin is a hepatic protein found to be raised in patients with ACD and reduced in IDA. Inflammatory cytokines are the most important triggers for hepcidin production. Hepcidin affects iron homeostasis by inhibition of a divalent iron transporter protein-1, that in turn hinders enteral iron absorption, and blocking a ferroportin, this inhibits release of iron from iron stores. Both causes sideropenia and raised iron levels in the reticulo-endothelial tissues [9, 10].
Splenomegaly was reported in patients with SAs. In IDA, splenomegaly was described with Paterson-Kelly syndrome and hypopituitarism, whereas in ACD, it is a diagnostic feature of the underlying disease. The classic triad of Paterson-Kelly syndrome is retropharyngeal dysphagia, esophageal web, and iron deficiency anemia [11-14].
This study was conducted to evaluate the frequency, and clinical significance (diagnostic/prognostic) of splenomegaly in patients with SAs, and also to assess the association between different grades of splenomegaly and both clinical and hematological profiles of patients.
| Materials and Methods | ▴Top |
Study design and subjects
A prospective longitudinal study was conducted at the Department of Internal Medicine, Assiut University Hospital over a period of 6 months. Two groups of patients were enrolled in the study, patients with IDA, patients with ACD, and another group of gender- and age-matched healthy volunteers was included as controls. Patients were recruited among those who were admitted or attending the outpatient clinics of Internal Medicine Department, while controls were among students, staff and co-workers. Consents of patients and controls were obtained before enrollment in the study. However, as mentioned before, splenomegaly in ACD is related to the underlying etiology, accordingly the study focused on patients with IDA. Hence, the study participants were subgrouped into group 1: patients with IDA, group 2: sideropenic control (patients with ACD), and group 3: normal controls.
Methods
Data collection
Demographic and clinical data of the study groups were obtained through detailed medical history and clinical examination, with particular stress on dietary habits and nutritional history, and also detailed menstrual history was obtained in females. Hematological profiles were obtained from results of laboratory investigations.
Patients with splenomegaly were asked for regular follow-up at the outpatient clinic every 2 weeks, and in each follow-up visit, patients’ splenic sizes were reassessed clinically together with laboratory assessment of anemia.
Diagnosis of SAs in the study groups
Diagnosis of SAs was accomplished by presence of general symptoms and signs suggestive of anemia. Specific signs such as smooth tongue, flattened nails, angular cheilitis, and koilonychias were suggestive of IDA [15]. Presence of chronic infection, inflammation, or malignancy was suggestive of ACD. Diagnosis of SAs was ascertained by laboratory detection of blood hemoglobin (Hb) level < 11.8 g/dL in females and < 13.8 g/dL in males.
Hematologically, presence of microcytosis (MCV < 80 fL), hypochromia (MCHC < 32 g/dL), sideropenia (serum iron < 50 μg/dL) and impaired reticulocytic response to anemia was diagnostic of SAs in the study subjects. Normocytic, normochromic anemia and reduced TIBC were diagnostic of ACD, while raised TIBC was present in IDA. Blood film with target cells or pencil shaped poikilocytes was highly suggestive of IDA. Patients with dimorphic blood film were excluded from the study.
In patients with microcytic hypochromic anemia and splenomegaly, Hb electrophoresis was performed to exclude thalassemia minor or trait. Bone marrow aspirate was performed in selected cases to exclude hypersplenism and differentiate IDA from ACD. In presence of reticulocytosis, direct antiglobulin test was done.
Diagnosis of the etiology of SAs in the study patients
Various laboratory, radiological and histopathological investigations were performed in a trial to verify the underlying etiology of SAs in the study groups. These included thorough nutritional history, stool and urine analyses, erythrocytic sedimentation rate (ESR), C-reactive protein (CRP), KFT and LFT. Abdominal or pelvic ultrasound and upper or lower endoscope were also performed as indicated.
Assessment of splenomegaly in patients with SAs
Splenomegaly was assessed in the study groups by thorough clinical history and examination. On detailed clinical examination, splenomegaly was considered by detection of dull Traube’s area or palpable spleen either in supine or right lateral positions. On our practice, clinical examination of patients attending the outpatient clinics or admitted in the ward usually takes place early in the morning before patients have their breakfast; however, some of patients have their breakfast before examination. All patients were examined by the hematology resident in charge before the researcher. Grading of splenomegaly was mainly based on the WHO proven Hackett’s clinical grading as follows [16]: Class 0: impalpable spleen; Class 1: just palpable spleen only with deep inspiration; Class 2: palpable spleen but not below a horizontal line passing half way between the costal margin and umbilicus; Class 3: palpable spleen but not below a horizontal line passing through the umbilicus; Class 4: palpable spleen but not below a horizontal line between the umbilicus and pubic symphysis; and Class 5: palpable spleen beyond class 4. Splenomegaly was diagnosed mild, moderate or massive if it is Hackett’s class 1 and 2, 3, 4, and 5, respectively. Confirmation of presence or absence of splenomegaly was done with the least hazardous radiographic assessment tool, abdominal ultrasound (US).
Abdominal US was performed using an Ultrasound System (GE, LOGIQ 3 Color Doppler) for all patients using 3.5 - 5.0 MHz convex transducer. The splenic size was measured (in cm) with the probe in the left upper quadrant. The largest anteroposterior dimension of the spleen was identified and measured. US scoring system was used to evaluate the edge, surface and parenchymal texture of the spleen. Score 0 means normal and score 1 means splenomegaly that was defined as an anteroposterior dimension > 13 cm, without any abnormality of the structure [17].
Treatment of the study groups
Treatment of SAs included treatment of the underlying etiology of SA. Those with IDA received ferrous fumarate tablets 200 mg Tds immediately after meals together with vitamin C supplementation and were advised for consuming iron rich diets. In ACD, erythropoietin and iron supplementation were provided. Intravenous iron and packed RBC transfusions were used to treat those with severe anemia and those intolerant to oral iron supplements [18-22]. Anemia was considered mild if Hb > 10 g/dL, moderate if Hb 7 - 10 g/dL and severe if Hb < 7 g/dL.
Follow-up for the study groups
Patients with evident splenomegaly were asked for regular follow-up at the hematology outpatient clinic firstly after 10 days and then every 2 weeks until Hb reached near normal values within 2 - 3 months. In the first visit, assessment of response to treatment was evaluated by the rising reticulocyte count (Retic). In each visit, patients were reassessed clinically, and with laboratory investigations. Abdominal US was repeated in the last follow-up visit. Data were recorded in a hand written follow-up file available at the clinic for each patient. Patients with IDA were advised to continue treatment for 6 months after Hb reached normal values to replenish iron stores.
Ethical considerations
The study aims and methodology were discussed to patients and controls; furthermore they were consistent with the World Medical Association (WMA) Declaration of Helsinki for ethics in medical research [23]. Consent for participation in the study was obtained from both patients and controls. Patients were asked to feel free to withdraw from the study at any time.
Statistical analysis
Data were collected, and then introduced into a personal computer substituting patients’ names with code numbers. The collected data were analyzed with Graphpad Prism V5, Italy and SPSS V. 17 software (SPSS Inc., Chicago, TL, USA). Quantitative variables were expressed as mean ± SD, median, and range while qualitative variables were expressed as percentages from the total number. The one-way ANOVA and Tukey’s multiple comparison tests were used to compare means, while the Chi-square test was used to analyze differences among qualitative variables among the study groups.
Sensitivity, specificity, positive, and negative predictive values with 95% confidence intervals were reported for US in detecting splenomegaly in the patients taking Hackett’s clinical grading.
| Results | ▴Top |
Characteristics of the study population
Demographic and clinical characteristics of the study groups
A total of 108 participants were included in the study, of whom 53 were with IDA (group 1), 30 were with sideropenic controls (group 2) and 25 were healthy controls (group 3). The mean ages were 30.89 ± 13.39, 31.21 ± 15.15 and 31.01 ± 14.01 years, respectively (P = 0.912). Gender analysis showed female predominance in the study patients with male to female ratio 1:1.1 in IDA and 1:1.5 in sideropenic controls. The vast majority of the study participants were from Assiut governorate, 56.5%. Due to perfect matching, there were no significant differences in age, gender and residential distribution among the study groups. SAs were more common in rural residents compared with urban residents (59.3% vs. 39.8 %). Of the patients with IDA, 47.2% were singles, while 83.3% of sideropenic controls were parents. IDA was more common in students and housewives (28.3% and 26.4%, respectively).
The most common presenting complaints were dizziness in those with IDA (98.2%), while non-hematological manifestations were more common in sideropenic controls (60%). One patient with IDA (1.8%) presented with delayed puberty. More than two-thirds (69.8%) of group 1 patients were excessive drinkers of tea vs. 50% and 36% in groups 2 and 3, respectively. Malnutrition was documented in 22.6% and 20% of groups 1 and 2 patients, respectively. Specific features of IDA as angular stomatitis and koilonychia were present in 45.3% and 32.1% of group 1, respectively. Of group 1 or 2, 3.8% of patients were with hepatomegaly. Table 1 shows demographic and clinical characteristics of the study groups.
 Click to view | Table 1. Demographic and Clinical Characteristics of the Study Groups (Total n = 108) |
Hematologic and disease characteristics of the study groups
Expectedly hypochromia, microcytosis, thrombocytosis and raised TIBC were present in group 1 patients, on the contrary, normocytic, normochromic anemia with decreased TIBC and raised ESR and CRP were the most common features of group 2. Sideropenia was the unique feature of all the study patients. There was no significant difference in white blood cell (WBC) count among the study groups. There were significant differences in Hb, platelets (Plts), MCV S. iron and TIBC between group 1 patients and the controls, when considering group 2, differences were significant in Hb, Retic, S. iron, TIBC, ESR and CRP as depicted in Tables 2 and 3. Of group 1 patients, 47.2%, 52.8% and 0% were with severe, moderate and mild anemia, vs. 13.3%, 80%, and 6.7% in group 2, respectively. Interestingly mild anemia was detected in 20% of the healthy controls with a minimum Hb level of 11.5 g/dL as in Table 4.
 Click to view | Table 2. Laboratory and Hematologic Characteristics of the Study Groups (Total no = 108) |
 Click to view | Table 3. Tukey Multiple Comparison Test of Quantitative Variables of Patients With SAs Compared With the Controls (Total n = 108) |
 Click to view | Table 4. Degree and Treatment Modalities of SAs in the Study Patients (total n = 83) |
Underlying etiology of SA in the study patients
The most prevalent causes of IDA were menorrhagia, hemophilia, unknown etiology and occult bleeding, while those for ACD were chronic renal failure (CRF), rheumatoid arthritis, malignancy and systemic lupus erythematosus in descending order. Benzidine test was positive in 9.4% of patients with IDA, denoting occult blood in stools as in Figure 1.
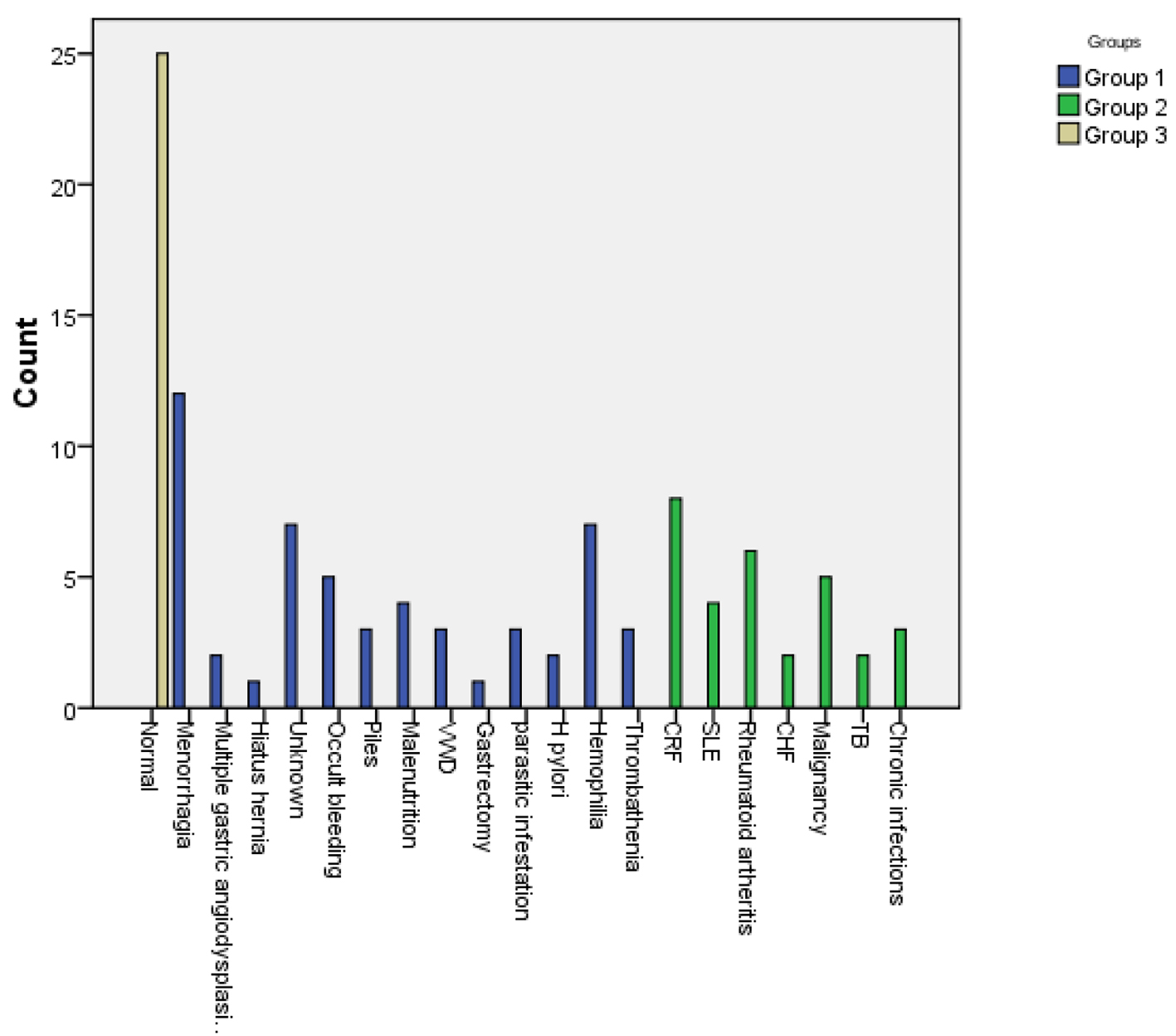 Click for large image | Figure 1. Causes of sideropenic anemias in the study patients. |
Splenomegaly in patients with IDA compared with the sideropenic and healthy control subjects
Splenomegaly was present in 11.3%, 40% and 0% of groups 1, 2 and 3, respectively. In IDA, half most of the patients were with Hackett’s G1 (9.4%) and only 1.9% with G2, and also in ACD, G1 comprised 33.3% followed by grade 0 (16.7%) and lastly grade 2 (6.7%). However, splenomegaly was sonographically proven only in five patients of group 1 (9.4%), and 10 patients in group 2 (33.3%). Table 1 shows distribution of splenomegaly in the study groups, and Table 5 shows diagnostic performance of US variables in predicting splenomegaly among groups 1 and 2.
 Click to view | Table 5. Diagnostic Performance of US Variables in Predicting Splenomegaly Among Groups 1 and 2 |
Splenomegaly in patients with IDA was more common in males (80%) and rare in those from urban community (20%). Their medical history denoted insufficient dietary intake of iron, pica and malnutrition. They were excessive drinkers of tea (100%) with angular stomatitis (80%), and koilonychias (100%). Hepatomegaly was associated with splenomegaly in 0% and 90% of patients with IDA and sideropenic controls, respectively.
When we correlated hematological parameters with grading of splenomegaly in patients with IDA, they were negatively associated with Hb, MCV, and Retic, and all patients were with severe anemia (Hb ranged from 3.2 to 6 g/dL). Table 6 shows factors associated with splenomegaly in patients with IDA.
 Click to view | Table 6. Factors Associated With Splenomegaly in Patients With IDA (Total no = 53) |
The stool analyses showed Giardia lamblia cysts in two patients with IDA and splenomegaly, hookworm ova in one patient and occult bleeding in one patient. The possible etiology of IDA in the other patient was unknown; however, the defective dietary intake of iron was marked in all patients and was continuous for many years.
Follow-up of patients with SAs and splenomegaly after treatment revealed gradual progressive reduction of splenic size with increasing Hb. After 3 months follow-up spleen was nearly impalpable in those with grade 1 Hackett’s; however, dullness at Traube’s area was still detected in patient with grade 2. Splenomegaly was still sonographically detected in two patients. These findings were noted on patients with IDA (group 1). On the contrary, splenic size remained stable in the sideropenic control patients (group 2).
| Discussion | ▴Top |
SAs are hypoproliferative anemias caused by iron deficiency and/or decreased erythropoietin (EPO) production and/or reduced response to EPO. The later is due to resistance of target cells to EPO action or reduced number of cells [24]. This study was conducted to elucidate splenomegaly in patients with SAs in terms of occurrence, clinical and hematological profile and the effect of treatment on splenomegaly. The study focused on IDA, while ACD was used as a sideropenic control.
In this study, SAs were more common in females; IDA was more prevalent in rural residence, while urbanization was obvious in sideropenic controls. Furthermore, IDA was more common in students and housewives, while ACD was in those who were on regular office work. These expected results were explained by increased prevalence of iron deficiency and chronic inflammatory diseases in females and higher vegans in rural communities [25, 26].
This study confirmed direct relationship between excessive intake of tea and incidence of IDA. A common custom in Egypt is to drink nearly 2 g/250 mL of red tea right after each meal particularly after lunch. Numerous studies reported that tea hinders iron absorption and advised tea drinkers to have their cups at least 1 h after meal [27-29].
Manifestations of tissue iron deficiency were much higher than that in comparable studies; this could be explained by the longer duration of iron deficiency in our patients. However, results of this study were accordant with others in showing positive association between the degree of tissue iron deficiency and severity of IDA and revealing that angular cheilitis was more prevalent than koilonychias. Both angular cheilitis and koilonychias were explained by deficiency of iron-based enzymes in the mucosal and epithelial tissues [30, 31].
In accordance with other studies, reduced Hb, MCV, MCHC, and thrombocytosis were the CBC features of IDA; on the contrary, normocytosis and normochromia were evident in sideropenic controls [32]. An interesting finding was laboratory detection of mild anemia in asymptomatic controls with the minimum Hb of 11.5 g/dL in females. This finding denoted that the lower cutoff value of Hb should be tailored for each population specifically.
In this study, the most common etiology of IDA was menorrhagia, while CRF was the commonest cause of ACD. This study confirmed the findings of others that IDA could retard growth and development in children, and also reaffirmed that gastrointestinal tract blood loss and H. pylori infections are common causes of IDA. As reported by others, hiatus hernia was the underlying etiology of IDA in one of our patients [33-36]. Although the recommended daily requirements of iron are very small, IDA due to ineffective dietary intake was noted in our patients, explaining that most of them were from rural community. Accordingly, the main elements of their diets were milk and milk products; fruits and vegetables both are poor sources for iron.
Splenomegaly was detected in approximately one-tenth of patients with IDA; this was albeit consistent and inconsistent to other studies. Unlike other studies, there was no detectable splenomegaly in the control group [37-39].
When considering the sideropenic control group, splenomegaly was detected in more than half of the patients and was closely related to the underlying etiology of anemia; furthermore, heptosplenomegaly was evident in a considerable proportion of patients. However, there was no association between the degree of anemia and Hackett’s grading of splenomegaly in the sideropenic control group. As reported by others, treatment of the underlying etiology of anemia improved hematological profile of the patient [18, 40, 41]; however, it did not affect splenic size. This denoted that the etiopathogenesis of splenomegaly in the sideropenic controls is not related to the anemia itself.
In this study, patients with IDA and splenomegaly were mostly from rural community and the vast majority of them were males in their late teens or early twentieth. Their nutritional history denoted ineffective dietary supply of iron and pica. Parasitic infestation was detected in three of the patients. They were suffering from IDA for years, with periods of interrupted iron supplementation. Concomitant with other studies, splenomegaly was impalpable or mild to moderate (Hackett’s grades 1 and 2) in most of the patients. Furthermore, the degree of splenomegaly was positively correlated with the severity of anemia, which was consistent with Hussain et al and inconsistent with Dabadghao and his coworkers [14, 42, 43]. Notably, this study showed reduction in splenic size with correction of Hb. Another important finding was the strong association between splenomegaly and kiolonychia in patients with IDA; kiolonychia is a sign of severe long standing IDA [30]. Expectedly, the degree of splenomegaly will be directly correlated with both the severity and duration of IDA, and this assumption was proved with the results of the current study.
The etiopathogenesis of splenomegaly in patients with IDA is still unclear. However, the most acceptable explanation is that IDA is a hypoproliferative anemia with poikilocytosis and anisocytosis both leading to splenic hyperplasia. Another explanation is extramedullary hematopoiesis, which could in turn explain the rare association of hepatomegaly and splenomegaly in patients with IDA. The later assumption could explain the association of splenomegaly with duration and severity of IDA; furthermore, it explained resolution of splenomegaly with correction of IDA. Although malnutrition was detected in our patients, it could not explain the occurrence of splenomegaly in IDA as it was a micronutrient malnutrition rather than a protein energy malnutrition [44]. However, the presence of giardiasis in two of our patients could be a contributing factor for development of splenomegaly in them [45].
Conclusion and recommendations
In conclusion, the current study demonstrated that splenomegaly had considerable clinicohematologic significance in patients with SAs. Hackette’s grade 1 or 2 splenomegaly was a common finding in patients with severe, chronic IDA that was mainly caused by malnutrition. Furthermore, iron supplementation and replenishment of iron stores led to gradual resolution of splenomegaly. This denoted that splenomegaly, in patients with IDA, is a very simple clinically based diagnostic/prognostic index. However, every effort has to be done to exclude thalassemia in patients with microcytic hypochromic anemia and splenomegaly before prescribing iron supplementation. This was not the case in patients with ACD where splenomegaly was found to be related to the underlying etiology of anemia rather than the anemia itself.
Based on the findings of this study, we recommended reducing tea drinking particularly in children and females who are already on a state of negative iron balance due to increased demands. Furthermore, we advice people to avoid drinking tea after the main meal where a great proportion of iron requirements are supplied.
Acknowledgments
The authors wish to express deep thanks and gratitude to the entire control group who volunteered participation in the study for the seeking of robust medical knowledge.
Competing Interests
The authors have no competing interests.
Author Contributions
Dr. Safaa A. A. Khaled found out the research problem, formulated the study objectives, put the research plan, collected data, did the statistical analyses and edited the paper. Dr. Gehan S. Siefeldien did the radiographic assessment, collected and interpreted radiographic data, analyzed it and edited the radiographic section of the paper. Both authors revised the article before submission.
| References | ▴Top |
- Cavill I, Auerbach M, Bailie GR, Barrett-Lee P, Beguin Y, Kaltwasser P, Littlewood T, et al. Iron and the anaemia of chronic disease: a review and strategic recommendations. Curr Med Res Opin. 2006;22(4):731-737.
doi pubmed - Dallman PR, Yip R, Johnson C. Prevalence and causes of anemia in the United States, 1976 to 1980. Am J Clin Nutr. 1984;39(3):437-445.
pubmed - WHO Global Database on Anemia. Worldwide Prevalence of anemia 1993-2005. Geneva, Switzerland: World Health Organization, 2008.
- Alvarez-Hernandez X, Liceaga J, McKay IC, Brock JH. Induction of hypoferremia and modulation of macrophage iron metabolism by tumor necrosis factor. Lab Invest. 1989;61(3):319-322.
pubmed - Cazzola M, Ponchio L, de Benedetti F, Ravelli A, Rosti V, Beguin Y, Invernizzi R, et al. Defective iron supply for erythropoiesis and adequate endogenous erythropoietin production in the anemia associated with systemic-onset juvenile chronic arthritis. Blood. 1996;87(11):4824-4830.
pubmed - Ferrucci L, Semba RD, Guralnik JM, Ershler WB, Bandinelli S, Patel KV, Sun K, et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood. 2010;115(18):3810-3816.
doi pubmed - Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347-360.
doi pubmed - Busbridge M, Griffiths C, Ashby D, Gale D, Jayantha A, Sanwaiya A, Chapman RS. Development of a novel immunoassay for the iron regulatory peptide hepcidin. Br J Biomed Sci. 2009;66(3):150-157.
doi pubmed - Skikne BS, Punnonen K, Caldron PH, Bennett MT, Rehu M, Gasior GH, Chamberlin JS, et al. Improved differential diagnosis of anemia of chronic disease and iron deficiency anemia: a prospective multicenter evaluation of soluble transferrin receptor and the sTfR/log ferritin index. Am J Hematol. 2011;86(11):923-927.
doi pubmed - Brasse-Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, Beaumont C. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology. 2011;140(4):1261-1271 e1261.
- Hoffman RM, Jaffe PE. Plummer-Vinson syndrome. A case report and literature review. Arch Intern Med. 1995;155(18):2008-2011.
doi pubmed - Prasad AS, Halsted JA, Nadimi M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med. 1961;31:532-546.
doi - King R, Mizban N, Rajeswaran C. Iron deficiency anaemia due to hypopituitarism. Endocrine Abstr. 2009;19:278.
- Safaa A, Khaled A. Aggravation of Iron Deficiency Anemia after Hormone Replacement Therapy in a Patient with Hypopituitarism and Hepatosplenomegaly. Int J Adv Res Biol Sci. 2015;2(12):324-329.
- Shah A. Iron deficiency anemia - Part-II (etiopathogenesis and diagnosis). Indian J Med Sci. 2004;58(3):134-137.
pubmed - Ogilvie C. Evans CC. Splenomegaly. In: Wratt G. Symptoms and signs in Tropical Disease. Chamberlaine's symptoms and signs in Clinical medicine: 12th edition. Butterworth-Heineman. 1997;315-324.
- Afzal S, Masroor I, Beg M. Evaluation of Chronic Liver Disease: Does Ultrasound Scoring Criteria Help? Int J Chronic Dis. 2013;2013:326231.
- Doyle MK, Rahman MU, Han C, Han J, Giles J, Bingham CO, 3rd, Bathon J. Treatment with infliximab plus methotrexate improves anemia in patients with rheumatoid arthritis independent of improvement in other clinical outcome measures-a pooled analysis from three large, multicenter, double-blind, randomized clinical trials. Semin Arthritis Rheum. 2009;39(2):123-131.
doi pubmed - Liu K, Kaffes AJ. Iron deficiency anaemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24(2):109-116.
doi pubmed - Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60(10):1309-1316.
doi pubmed - Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, Balan S, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol. 2004;22(7):1301-1307.
doi pubmed - Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematology Am Soc Hematol Educ Program. 2010;2010:338-347.
doi - World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194.
doi pubmed - Faquin WC, Schneider TJ, Goldberg MA. Effect of inflammatory cytokines on hypoxia-induced erythropoietin production. Blood. 1992;79(8):1987-1994.
pubmed - Kim JY, Shin S, Han K, Lee KC, Kim JH, Choi YS, Kim DH, et al. Relationship between socioeconomic status and anemia prevalence in adolescent girls based on the fourth and fifth Korea National Health and Nutrition Examination Surveys. Eur J Clin Nutr. 2014;68(2):253-258.
doi pubmed - Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med. 2006;160(11):1108-1113.
doi pubmed - Nelson M, Poulter J. Impact of tea drinking on iron status in the UK: a review. J Hum Nutr Diet. 2004;17(1):43-54.
doi pubmed - Disler PB, Lynch SR, Charlton RW, Torrance JD, Bothwell TH, Walker RB, Mayet F. The effect of tea on iron absorption. Gut. 1975;16(3):193-200.
doi pubmed - Kaltwasser JP, Werner E, Schalk K, Hansen C, Gottschalk R, Seidl C. Clinical trial on the effect of regular tea drinking on iron accumulation in genetic haemochromatosis. Gut. 1998;43(5):699-704.
doi pubmed - Kumar G, Vaidyanathan L, Stead LG. Images in emergency medicine. Koilonychia, or spoon-shaped nails nails, is generally associated with iron-deficiency anemia. Ann Emerg Med. 2007;49(2):243, 250.
- Uchida T, Matsuno M, Ide M, Kawachi Y. [The frequency and development of tissue iron deficiency in 6 iron deficiency anemia patients with plummer-vinson syndrome]. Rinsho Ketsueki. 1998;39(11):1099-1102.
pubmed - Yates JM, Logan EC, Stewart RM. Iron deficiency anaemia in general practice: clinical outcomes over three years and factors influencing diagnostic investigations. Postgrad Med J. 2004;80(945):405-410.
doi pubmed - Bandhu R, Shankar N, Tandon OP. Effect of iron on growth in iron deficient anemic school going children. Indian J Physiol Pharmacol. 2003;47(1):59-66.
pubmed - Soliman AT, Al Dabbagh MM, Habboub AH, Adel A, Humaidy NA, Abushahin A. Linear growth in children with iron deficiency anemia before and after treatment. J Trop Pediatr. 2009;55(5):324-327.
doi pubmed - Rockey DC, Cello JP. Evaluation of the gastrointestinal tract in patients with iron-deficiency anemia. N Engl J Med. 1993;329(23):1691-1695.
doi pubmed - Windsor CW, Collis JL. Anaemia and hiatus hernia: experience in 450 patients. Thorax. 1967;22(1):73-78.
doi - Nadeem A, Ali N, Hussain T, Anwar M. Frequency and etiology of splenomegaly in adults seeking medical advice in Combined Military Hospital Attock. J Ayub Med Coll Abbottabad. 2004;16(4):44-47.
pubmed - Sundaresan JB, Dutta TK, Badrinath S, Jagdish S, Basu D. A hospital-based study of splenomegaly with special reference to the group of indeterminate origin. J Indian Med Assoc. 2008;106(3):150, 152, 154 passim.
pubmed - McIntyre OR, Ebaugh FG. Palpable spleen in college freshmen. Ann Intern Med. 1967;66:30-33.
doi - Ali N, Anwar M, Ayyub M, Nadeem M, Ejaz A, Qureshi AH, Qamar MA. Hematological evaluation of splenomegaly. J Coll Physicians Surg Pak. 2004;14(7):404-406.
pubmed - Papadaki HA, Kritikos HD, Valatas V, Boumpas DT, Eliopoulos GD. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following antitumor necrosis factor-alpha antibody therapy. Blood. 2002;100:474-482.
doi pubmed - Hussain I, Ahmed I, Mohsin A. Causes of splenomegaly in adult local population presenting at tertiary care centre in Lahore. Pakistan J Gastroenterol. 2002;16(1):12-16.
- Dabadghao VS, Diwan AG, Raska AM. A Clinicohaematological Profile of Splenomegaly. Bombay Hospital Journal. 2012;54:10-17.
- Anwer I, Awan JA. Nutritional status comparison of rural with urban school children in Faisalabad District, Pakistan. Rural Remote Health. 2003;3(1):130.
pubmed - Charalabopoulos K, Charalabopoulos A, Papadopoulou CH, Papalimneou V. Giardia lamblia intestinalis: a new pathogen with possible link to Kikuchi-Fujimoto disease. An additional element in the disease jigsaw. Int J Clin Pract. 2004;58(12):1180-1183.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


