| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 7, Number 2, May 2018, pages 79-82
A Rare t(5;11)(q35;q13) Translocation in an Elderly Patient With Acute Myeloid Leukemia With Maturation: A Case Report
Masahiro Manabea, c, Yuuji Hagiwarab, Reiko Asadab, Dai Momosea, Yasuyoshi Suganoa, Ki-Ryang Koha
aDepartment of Hematology, Osaka General Hospital of West Japan Railway Company, 1-2-22 Matsuzaki-cho, Abeno-ku, Osaka 545-0053, Japan
bDepartment of Clinical Laboratory, Osaka General Hospital of West Japan Railway Company, 1-2-22 Matsuzaki-cho, Abeno-ku, Osaka 545-0053, Japan
cCorresponding Author: Masahiro Manabe, Department of Hematology, Osaka General Hospital of West Japan Railway Company, 1-2-22 Matsuzaki-cho, Abeno-ku, Osaka 545-0053, Japan
Manuscript submitted March 14, 2018, accepted April 10, 2018
Short title: AML With t(5;11)(q35;q13)
doi: https://doi.org/10.14740/jh394w
| Abstract | ▴Top |
The t(5;11)(q35;q13) reciprocal translocation is a rare chromosomal abnormality that can arise in myeloid neoplasms, mainly in children and younger adults. Here, we report a case of acute myeloid leukemia with maturation, involving an 85-year-old, in which the tumor cells harbored the t(5;11)(q35;q13) chromosomal abnormality. We also address the diagnostic and immunophenotypic characteristics of acute myeloid leukemia involving t(5;11)(q35;q13), along with a review of the literature.
Keywords: t(5; 11)(q35; q13); Acute myeloid leukemia with maturation; Elderly
| Introduction | ▴Top |
Chromosomal examinations are important for diagnosing hematological neoplasms because of the prognostic significance of their findings. Myeloid malignancies containing the t(5;11)(q35;q13) translocation are very rare, and only four cases involving children or young adults have been reported [1-4]. Here, we present the case of an elderly patient with acute myeloid leukemia (AML) with maturation involving the t(5;11)(q35;q13) translocation.
| Case Report | ▴Top |
An 85-year-old female was referred to us because of leukocytosis involving the appearance of blasts. She had no symptoms. On admission, her white blood cell count was 10,900/µL (blast cells, 13%; myelocytes, 0.5%; neutrophils, 66.5%; eosinophils, 3.5%; lymphocytes, 13.5%; monocytes, 3%). Her hemoglobin level was 12.2 g/dL, and her platelet count was 378,000/µL. The patient’s serum level of lactate dehydrogenase was 272 U/L. A bone marrow examination revealed a hypercellular bone marrow with a blast cell frequency of 54.8% (Fig. 1). Chromosomal analysis of the patient’s bone marrow cells showed the following karyotype: 46,XX,t(5;11)(q35;q13)[8]/46,XX[12] (Fig. 2). Surface marker analysis revealed that the blast cells were positive for CD7 (29.3%), CD56 (63.5%), CD13 (26.5%), CD33 (25.2%), CD34 (81.1%), and human leukocyte antigen (HLA)-DR (97.1%) and negative for CD2, CD3, CD4, CD5, CD8, CD10, CD19, CD20, CD16, and CD14. These findings were consistent with AML with maturation. Due to her poor performance status, she was administered supportive care alone and died of intracranial hemorrhaging 6 months after the initial diagnosis.
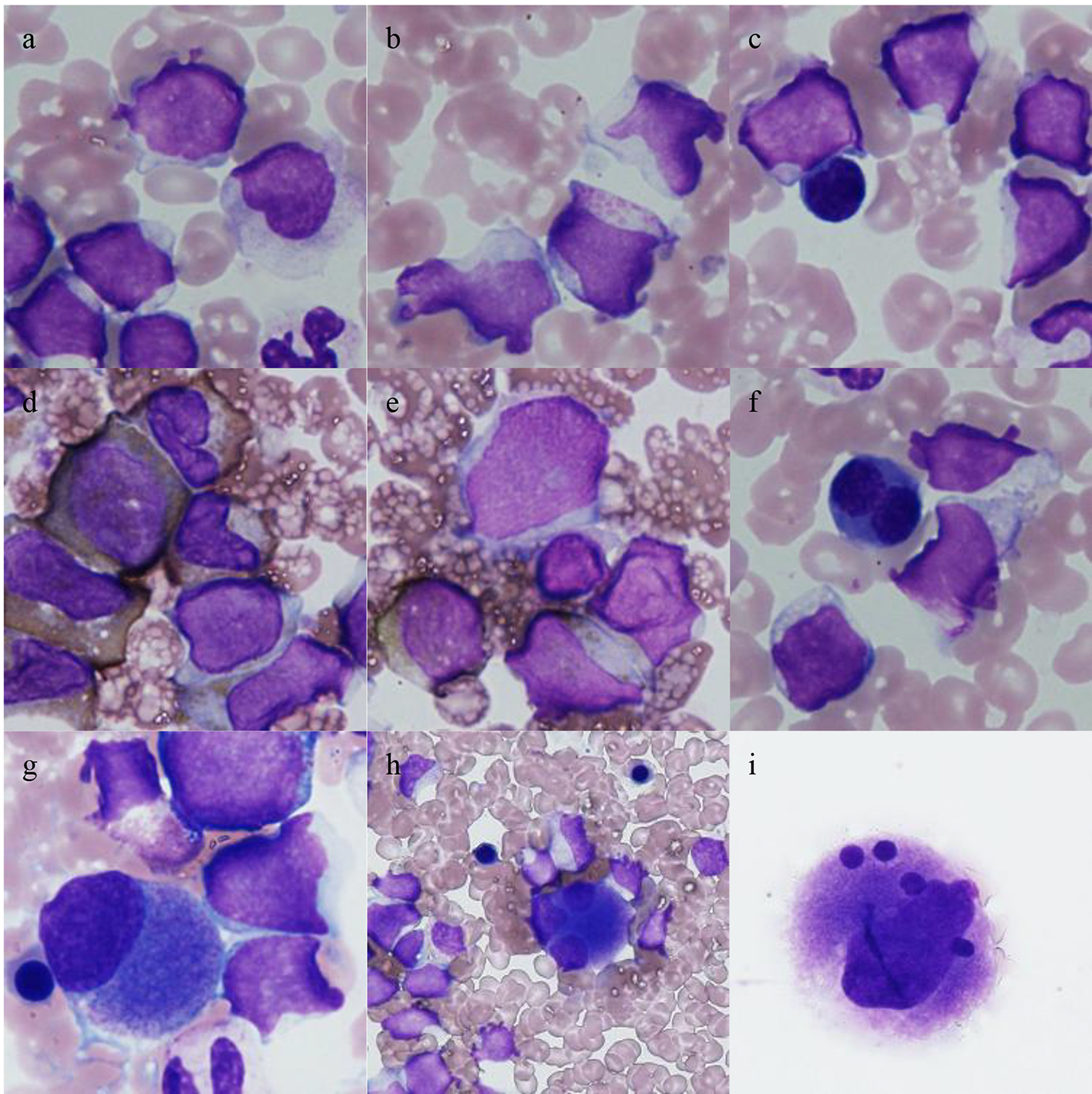 Click for large image | Figure 1. Bone marrow cells seen at the diagnosis of leukemia. Low frequencies of dyserythropoiesis and dysmegakaryopoiesis were observed. (a-c) Leukemic cells (May-Giemsa staining); abundant granules were occasionally observed (b); (d, e) blasts that were positively and negatively stained for myeloperoxidase, respectively; (f) a binuclear erythroblast (May-Giemsa staining); (g-i) megakaryocytes (May-Giemsa staining); (g) a small mononuclear megakaryocyte; (h) a trinuclear megakaryocyte; (i) a megakaryocyte containing multiple separate, round nuclei. |
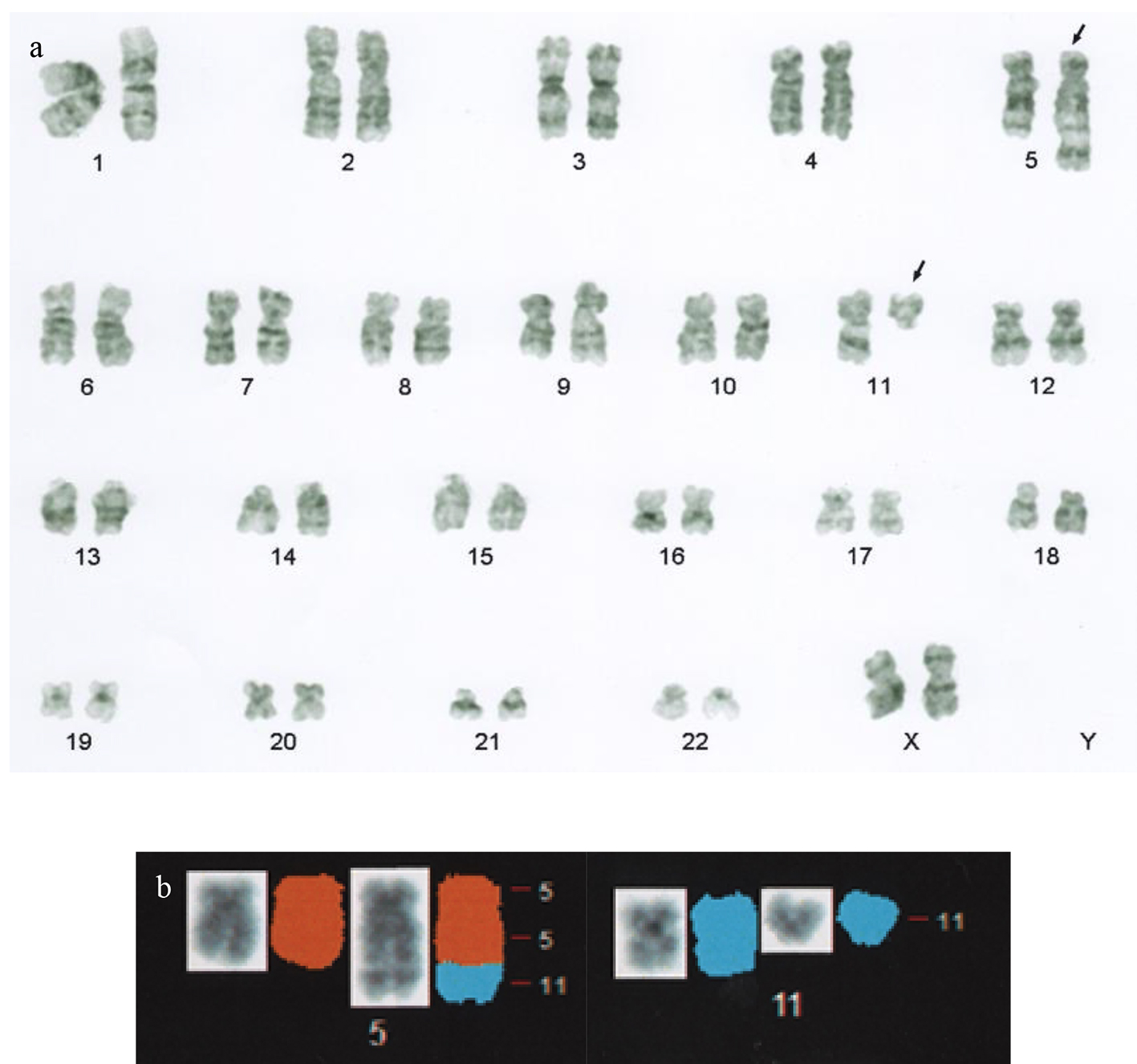 Click for large image | Figure 2. (a) G-band karyotype analysis performed at the diagnosis of leukemia revealed the following karyotype: 46,XX,t(5;11)(q35;q13). The arrows indicate derivative chromosomes. (b) Partial spectral karyotyping of the patient’s metaphase spread after spectrum-based classification (left, counterstained with 4’,6-diamino-2-phenylindole dihydrochloride; right, spectral karyotyping). |
| Discussion | ▴Top |
To the best of our knowledge, only four cases of AML involving the t(5;11)(q35;q13) translocation have been reported in the literature [1-4]. All of these cases involved children or young adults (Table 1 [1-5]). Among the reported cases, the present case involves the older patient. It has been shown that translocations involving the breakpoint 5q35, such as t(5;11)(q35;p15) or t(5;17)(q35;q21), mainly occur in AML cases involving children/adolescents/young adults [6]. In addition, it has been reported that the 11q13 translocation is more frequently found in children and adolescents with acute leukemia [7]. Hence, our case seems to be very rare as it involved an 85-year-old patient who harbored the t(5;11)(q35;q13) translocation, which consists of both 5q35 and 11q13.
 Click to view | Table 1. Cases of Acute Myeloid Leukemia Involving t(5;11)(q35;q13) |
Concerning the genes involved in this translocation, Wang et al reported that the nuclear receptor-binding SET domain protein (NSD1), which is located at 5q35, is not involved in this translocation, based on experiments involving the fluorescence in situ hybridization (FISH) technique [3]. Similarly, using FISH, de Oliveira et al showed that cyclin D1 (CCND1), which is located at 11q13, is also not involved in this translocation [4]. Although we could not perform a detailed analysis, e.g., ribonucleic acid sequencing, of the genetic rearrangements associated with this translocation due to a lack of specimens, it might be possible to determine the genetic characteristics responsible for this translocation through further case accumulation.
As for the diagnoses of the reported cases of AML involving the t(5;11)(q35;q13) translocation, there were three cases of AML with maturation, including our case (French-American-British classification; M2); one case of acute myelomonocytic leukemia (M4); and one case of acute monoblastic and monocytic leukemia (M5) (Table 1). Although de Oliveira et al reported that the 11q13 abnormality seemed to be associated with differentiation towards the monocytic lineage [4], M2 was the most common diagnosis among the reported cases. Hence, it cannot be assumed that the t(5;11)(q35;q13) translocation is restricted to the monocytic lineage.
Regarding the immunophenotypes of such cases, our case exhibited positivity for CD56. The CD56 antigen is expressed in natural killer/T-cell lymphoma, multiple myeloma, and AML. It was reported that CD56 is associated with a poorer prognosis, induction failure, and worse survival in AML [8-11]. In addition, CD56 expression is associated with recurrent chromosomal abnormalities, such as t(8;16)(p11;p13), t(8;21)(q22;q22), t(15;17)(q22;q21), and t(16;21)(p11;q22) [12]. The positivity rate of CD56 was 63.5% in our case. However, previous reports of t(5;11)(q35;q13)-harboring cases did not mention CD56 expression. Hence, it is unclear whether the CD56 expression seen in our patient is characteristic of t(5;11)(q35;q13) or was due to aberrant expression. The accumulation of further cases of t(5;11)(q35;q13)-harboring AML is necessary to obtain accurate data regarding the frequency of CD56 expression.
| References | ▴Top |
- Leverger G, Bernheim A, Daniel MT, Flandrin G, Schaison G, Berger R. Cytogenetic study of 130 childhood acute nonlymphocytic leukemias. Med Pediatr Oncol. 1988;16(4):227-232.
doi pubmed - Itoh M, Okazaki T, Tashima M, Sawada H, Uchiyama T. Acute myeloid leukemia with t(5;11): two case reports. Leuk Res. 1999;23(7):677-680.
doi - Wang TF, Horsley SW, Lee KF, Chu SC, Li CC, Kao RH. Translocation between chromosome 5q35 and chromosome 11q13 - an unusual cytogenetic finding in a primary refractory acute myeloid leukemia. Clin Lab Haematol. 2006;28(3):160-163.
doi pubmed - de Oliveira FM, Tone LG, Simoes BP, Falcao RP, Brassesco MS, Sakamoto-Hojo ET, dos Santos GA, et al. Acute myeloid leukemia (AML-M2) with t(5;11)(q35;q13) and normal expression of cyclin D1. Cancer Genet Cytogenet. 2007;172(2):154-157.
doi pubmed - Mitelman F, Johansson B, Mertens F, eds. Mitelman database of chromosome aberrations and gene fusions in cancer (2018). http://cgap.nci.nih.gov/Chromosomes/Mitelman. Accessed 10 Mar 2018.
- Johansson B, Harrison CJ. Acute myeloid leukemia. In: Heim S, Mitelman F, eds. Cancer Cytogenetics. 4th ed. Chichester: Wiley Blackwell. 2015; p. 62-125.
doi - Wong KF. 11q13 is a cytogenetically promiscuous site in hematologic malignancies. Cancer Genet Cytogenet. 1999;113(1):93-95.
doi - Baer MR, Stewart CC, Lawrence D, Arthur DC, Byrd JC, Davey FR, Schiffer CA, et al. Expression of the neural cell adhesion molecule CD56 is associated with short remission duration and survival in acute myeloid leukemia with t(8;21)(q22;q22). Blood. 1997;90(4):1643-1648.
pubmed - Raspadori D, Damiani D, Lenoci M, Rondelli D, Testoni N, Nardi G, Sestigiani C, et al. CD56 antigenic expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia. 2001;15(8):1161-1164.
doi pubmed - Di Bona E, Sartori R, Zambello R, Guercini N, Madeo D, Rodeghiero F. Prognostic significance of CD56 antigen expression in acute myeloid leukemia. Haematologica. 2002;87(3):250-256.
pubmed - Jekarl DW, Kim M, Lim J, Kim Y, Han K, Lee AW, Kim HJ, et al. CD56 antigen expression and hemophagocytosis of leukemic cells in acute myeloid leukemia with t(16;21)(p11;q22). Int J Hematol. 2010;92(2):306-313.
doi pubmed - Ikushima S, Yoshihara T, Matsumura T, Misawa S, Morioka Y, Hibi S, Imashuku S. Expression of CD56/NCAM on hematopoietic malignant cells. A useful marker for acute monocytic and megakaryocytic leukemias. Int J Hematol. 1991;54(5):395-403.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


