| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 7, Number 4, December 2018, pages 154-157
Hypercalcemia With Disseminated Osteolytic Lesions: A Rare Presentation of Adulthood Acute Lymphoblastic Leukemia
Shaimaa El-Ashwaha, c, Noha Eisaa, May Denewera, Yasmine Essama, Basma Atefa, Adel El-Badrawyb, Mohamed Mabeda
aThe Hematology Unit, Oncology Center, Mansoura University, Mansoura, Egypt
bDiagnostic and Interventional Radiology Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt
cCorresponding Author: Shaimaa El-Ashwah, The Hematology Unit, Oncology Center, Faculty of Medicine, Mansoura University, Egypt
Manuscript submitted August 3, 2018, accepted August 31, 2018
Short title: Hypercalcemia With Osteolytic Lesions in Adult B-ALL
doi: https://doi.org/10.14740/jh455w
| Abstract | ▴Top |
Osteolytic bone lesions and hypercalcemia without peripheral blasts B-cell acute lymphoblastic leukemia (B-ALL) are reported in children but rarely seen in adults. Herein, we described two patients with B-ALL presenting with hypercalcemia and symptomatic osteolytic bone lesions. They were treated by standard induction chemotherapy after correction of hypercalcemia with supportive measures. With this two case reports we would like to emphasize the importance of clinical awareness of hypercalcemia and osteolytic bone lesions as rare presentations of ALL. The prognostic implication of bone lesions and hypercalcemia in ALL is unclear and needs to be verified in large prospective studies. However, immediate recognition and treatment of hypercalcemia and the underlying B-ALL are vital since a delay of diagnosis poses a possible life-threatening risk.
Keywords: Acute lymphoblastic leukemia; Hypercalcemia; Osteolytic bone lesions
| Introduction | ▴Top |
Acute lymphoblastic leukemia (ALL), being the most common malignancy in children, is a relatively rare neoplasm in adults. ALL is more common in men and it accounts for about 15% of leukemias in adults. Patients with ALL usually present with fever, bone and joint pains, bleeding manifestations, lymphadenopathy or hepatosplenomegaly [1].
Bone osteolytic lesions and hypercalcemia are rare events in most hematologic malignancies, except in patients with multiple myeloma and in adult T-cell leukemia/lymphoma (ATLL) patients associated with human T-cell leukemia/ lymphoma virus-1. Only individual cases of osteolytic lesions in adult patients with ALL have been reported. Predictive prognostic significance of bone lesions is still unclear [2].
The incidence of hypercalcemia varied from 50% to 90% in ATLL, from 27% to 35% in lung cancer, from 25% to 30% in breast cancer, between 7% and 30% in multiple myeloma, less than 10% in malignant lymphoma and even less than 1% in leukemia patients [3].
Here, we report two cases with B-ALL who presented with multiple osteolytic bone lesions associated with hypercalcemia.
| Case Reports | ▴Top |
Case 1
A 28-year-old male presented on October 20th, 2011 with generalized bone aches, associated with intermittent fever and weight loss with no significant past medical history. His examination was unremarkable apart from pallor. Complete blood count (CBC) at presentation revealed WBC: 9.2 × 109/L without peripheral blood blasts, hemoglobin: 9.2 g/dL, MCV = 80, platelets: 194 × 109/L, serum creatinine was 3.8 mg/dL (0.6 - 1.3), serum uric acid: 14.5 mg/dL (2.6 - 7.2), serum calcium: 12.5 mg/dL (8.8 - 10.8), serum albumin 3.8 g/dL (3.5 – 5). Because of presence of B symptoms and unexplained anemia, bone marrow aspiration (BMA) was done and showed 95% blast cells with high nuclear cytoplasmic ratio, fine nuclear chromatin and inconspicuous nucleoli. The diagnosis of Philadelphia negative B-ALL was confirmed by immunophenotyping on bone marrow (BM) which revealed positivity of lymphoblasts for TdT, CD34, CD10, CD19, CD20, sIgM and negativity for CD7, CD2, CD3, CD13 and CD33. Real time-PCR for Philadelphia chromosome was negative. Cerebrospinal fluid (CSF) examination revealed no abnormal cells. Whole body skeleton computed tomography (CT) revealed multiple osteolytic bone lesions affecting most of dorsal, lumbar spine, symphysis pubis, iliac, pubic bones and sternum (Fig. 1). The patient received supportive treatment including hydration, Lasix and dexamthasone for hypercalcemia and started MRC-UK chemotherapy protocol for ALL after normalization of serum calcium level. Evaluation of response after phase I of treatment revealed complete hematological response with normocellular BM and 1% blast cells. At the same time, a new whole body skeleton CT revealed persistence of the same previously described lesions. Patient completed induction and consolidation of MRC-UK and started maintenance chemotherapy. The patient was still in hematological remission as seen on follow-up BM examination on April 27th, 2013. Two months later, his CBC showed leukocytosis with WBCs: 18.4 × 109/L, Hb: 14.1 g/dL and platelets: 145 × 109/L. BMA at that time showed picture of relapse with blast cell 35%. The patient went into a second complete response induced by salvage HAM chemotherapy protocol as revealed by BMA showed 1% blast cells. The patient has no histocompatible donor for an allogeneic hematopoietic stem cell transplant. Therefore, he received two additional HAM as consolidation and started maintenance therapy. Unfortunately, he developed pancytopenia and severe neutropenia that was complicated by pneumonia and septic shock and he died on February 17th, 2014.
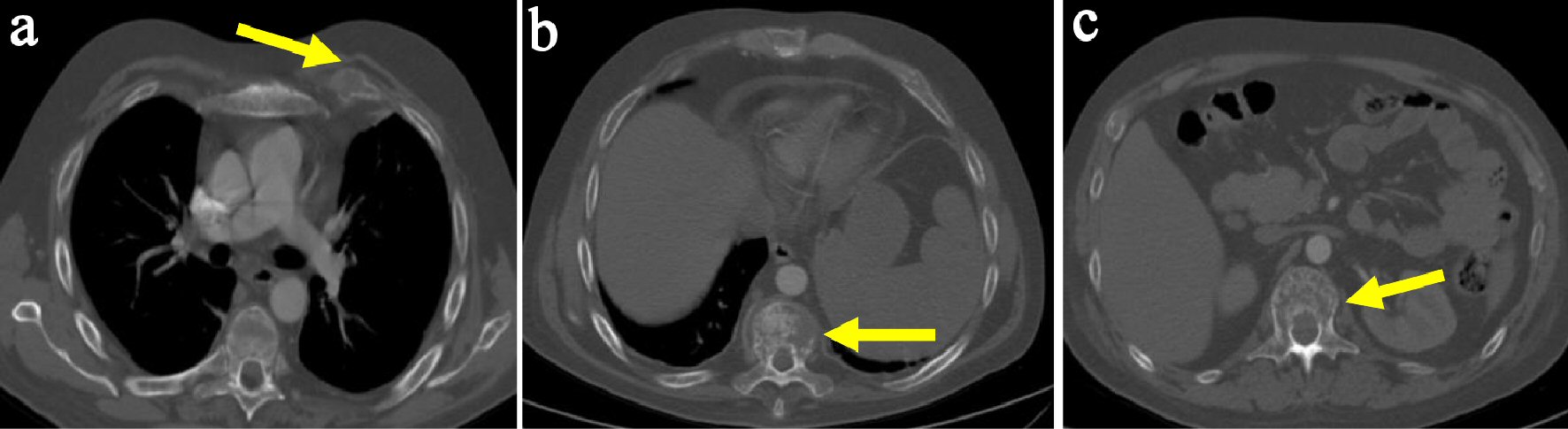 Click for large image | Figure 1. Male patient aged 25 years with ALL. MDCT scan (bone window) revealed multiple osteolytic bone lesions of left anterior rib (a), thoracic vertebra (b) and lumbar vertebra (c). |
Case 2
A 27-year-old female presented at the Urology Center, Mansoura University on June 21th, 2015 with history of repeated vomiting, frequent urination and generalized bone ache. Her examination was unremarkable apart from pallor. Laboratory investigations showed white blood cells: 7.3 × 109/L without peripheral blood blasts, hemoglobin: 8.9 g/dL, platelets: 140 × 109/L, serum creatinine: 1 mg/dL, serum uric acid: 5.9 mg/dL, serum calcium: 13.1 mg/dL, serum phosphorus: 4.1mg/dL, serum albumin: 3 g/dL with normal levels of parathyroid hormone. Patient received dexamethasone and Lasix and kept hydrated for the treatment of hypercalcemia and she was referred to our Hematology Unit to rule out multiple myeloma on June 23rd, 2015. After admission, the patient developed acute onset weakness of both lower limbs with headache and diplopia. Ophthalmological examination showed bilateral severe papilledema and restricted lateral rectus movement in left eye. Neurological examination revealed abducent nerve palsy and paraparesis. Laboratory parameters showed WBCs: 3.2 × 109/L without peripheral blood blasts, hemoglobin: 8.7 g/dL, platelets: 125 × 109/L, creatinine: 0.7 mg/dL, uric acid: 5.8 mg/dL, serum calcium: 10.98 mg/dL, serum albumin: 2.9 g/dL. CT brain was free apart from multiple osteolytic lesions affecting the skull bone. CSF examination revealed no abnormal cells. Whole body skeleton CT showed multiple osteolytic lesions affecting the scanned bones (both iliac bones, sacrum, the scanned vertebrae, sternum and skull bones) (Fig. 2). MRI brain revealed heterogeneous signal intensity in diplopic space of skull bones with meningeal thickening and enhancement supporting leukemic infiltration. BMA showed the picture of ALL-L2 with 95% blast cells. Flow cytometry confirmed the diagnosis of B-ALL with a population of blast cells positive for CD10, CD19, CD20, CD22, CD34 and negative for CD3, CD4, CD8, CD5, CD14, CD64, CD79, IgM, MPO, CD13, CD33 and CD117. Patient started Hyper CVAD chemotherapy protocol with triple intrathecal twice weekly and cranial irradiation. Patient received course I, but unfortunately the patient had septic shock and transferred to ICU where she was expired on July12th, 2015.
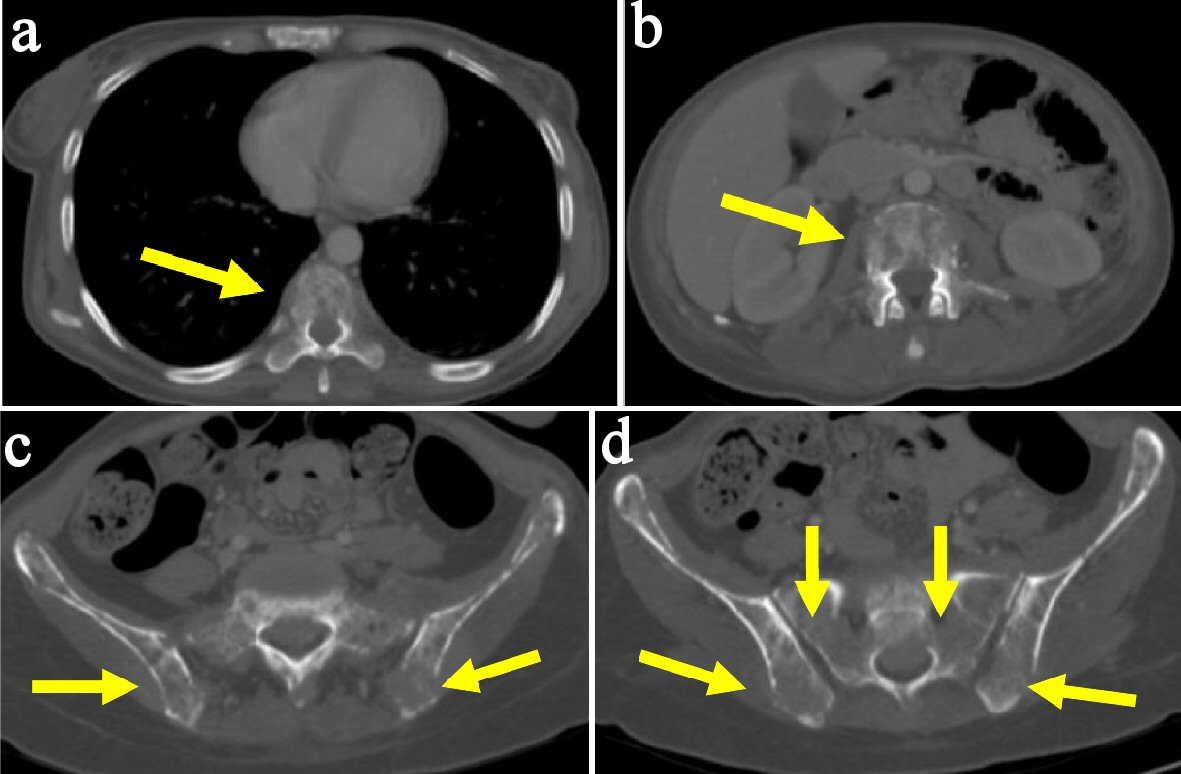 Click for large image | Figure 2. Female patient aged 27 years with ALL. MDCT scan (bone window) revealed multiple osteolytic bone lesions of thoracic vertebra (a), lumbar vertebra (b), both iliac bones and sacrum (c, d). |
| Discussion | ▴Top |
Few adult patients with osteolytic lesions and hypercalcemia in B-ALL have been reported [4]. We herein, report two patients with B-ALL presented with multiple osteolytic lesions with hypercalcemia and a normal white blood cell count without lymphoblasts in the peripheral blood.
Skeletal lesions can occur in various malignant hematological conditions. The mechanism leading to the lytic lesions and hypercalcemia noted in those patients remain uncertain. Probable causes of bone lesions may be involvement by tumor cells itself in leukemia, or mediated by parathormone-related peptide [5]. The first suggestion is local osteolytic skeletal metastases and possible cytokine release. In multiple myeloma or adult T cell leukemia/lymphoma, the primary mechanism of hypercalcemia is increased osteoclastic bone resorption by tumor cells (leukemic infiltration) in combination with local bleeding and osteonecrosis of the adjacent bone structures [6]. The second suggested mechanism in hematological malignancy is paraneoplastic production of humoral factors, mainly PTHrP, vitamin-D-like sterols, prostaglandin E2, TNF-α, and IL-6 [7]. Lymphoblasts in B-ALL have been shown to produce PTHrP. PTHrP stimulates bone resorption, renal calcium resorption and renal phosphate excretion via binding to the PTH/PTHrP-receptor. Higher serum levels have been detected in the majority of pediatric leukemic patients. On the other hand, PTHrP levels have been found to be normal in other reports, suggesting the importance of other humoral factors in this process [8]. Fukasawa et al demonstrated elevated serum concentrations of TNF-α, IL-6 and soluble IL-2 with normal concentration of PTHrP [9]. Other possible causes of hypercalcemia could be renal impairment as in our reported case. Since the kidney is the major excretory organ for calcium, it could inappropriately retain the large calcium load, which results from accelerated bone resorption. A possible mechanism of induced renal failure is the deposition of calcium in the kidney secondary to an increase in the calcium-phosphorus product [10].
The radiographic changes seen with leukemia include osteopenia, radiolucent metaphyseal bands, periosteal new bone formation and lytic or sclerotic lesions. Radiographic bone lesions at diagnosis have been reported in 41-70% of children with ALL [11]. However, destructive bone involvement with hypercalcemia in adults is uncommon [12]. Our second patient presented with many unusual findings like back pain and paraparesis, presence of multiple lytic lesions in the skull and no lymphadenopathy giving initial impression as a case of metastasis or multiple myeloma. Similarly, Verma et al diagnosed a 27-year-old female as Pre B-ALL who presented with paraparesis and multiple osteolytic lesions in skull [5].
Hypercalcemia is a life-threatening condition because of risk of cardiac arrhythmia, renal injury, acidosis, hypertension, dehydration and coma [13]. The prognostic implication of bone lesions in ALL is unclear [12]. Assessment of the outcome of our patients after chemotherapy showed disease-free survival of about 14 months for the first patient. The second patient died early during induction. The overall survival times were about 28 months for first patient and 20 days only for the second patient. Different outcome results have been reported by Kaiafa et al [1] and others [5, 8, 14]. Kaiafa et al reported a 24-year-old male patient with pre-B ALL. The patient finished Hyper-CVAD regimen and intrathecal infusions. Since an HLA-compatible donor could not be found, maintenance chemotherapy was administered, followed by complete remission for 23 months, until he presented with severe lumber pain. After reviewing laboratory, radiological and clinical findings, relapse of mixed phenotype acute leukemia was confirmed with multiple osteolytic lesions, hypercalcemia and mild renal function deterioration. FLAG-IDA (fludarabine, Aracytin, G-CSF, idarubicin) rescue therapy, and hydration plus furosemide, corticoids and bisphosphonates for hypercalcemia, were administered. After 6 days, the calcium level was normalized and accompanied with amelioration of his renal function and alleviation of the painful symptoms. One month after the first cycle of FLAG-IDA, he exhibited a second event of hypercalcemia and relapse of ALL with the same features as previously described, and he received the same treatment (second cycle of FLAG-IDA). Nonetheless, his general condition was deteriorated and he died soon after the episode due to severe sepsis [1]. Similarly, Chung et al 2011 described a 35-year-old male with ALL, Burkitt type presented with hypercalcemia and osteolytic lesion on the left mandibular body and ascending ramus area with severe vertical bone loss on the lower left posterior mandible. His calcium level did not decrease despite hemodialysis and intravenous infusion of Miacalcic. The patient showed aggressive course with sudden development of pneumonia, acute respiratory distress syndrome and was sent to the intensive care unit for ventilator care. Multiple organ failure developed with unstable blood pressure and the patient died within 7 days from diagnosis [14].
On the other hand, Granacher et al reported a 34-year-old male patient diagnosed with a Philadelphia chromosome positive precursor B-ALL. He was immediately treated with high dose corticosteroids and bisphosphonates to reduce hypercalcemia. He started imatinib (600 mg orally) and was subsequently treated with remission-induction chemotherapy consisting of prednisolone, daunorubicin, vincristine and PEG-L-asparaginase. There were no signs of cerebral invasion on CSF examination and the patient received two cycles of prophylactic intrathecal chemotherapy with methotrexate and dexamethasone during remission-induction therapy. He achieved complete molecular remission after the first induction chemotherapy and he eventually proceeded to maintenance chemotherapy [8].
Also, Verma et al reported a 27-year-old female with Pre B-ALL with central nervous system (CNS) involvement who presented with paraparesis and multiple osteolytic lesions in skull. Examination of neurological system revealed weakness of both lower limbs (power 3/5 in both limbs). Tone was normal and deep tendon reflexes were increased. Bilateral planters were extensor. Pain and temperature sensations were diminished (50%) below T6 spinal level. MRI spine showed lesions in dorsal subarachnoid space compressing spinal cord from D4 to D8 level. Patient was started on GMALL protocol with triple intrathecal therapy. The weakness in both lower limbs started improving within 48 h, so local site radiotherapy was not considered. Over 2 weeks period power improved to the extent of 4/5 with therapy. Post induction BM examination was in remission and CSF was clear. Patient continued consolidation chemotherapy and she was doing well [5].
Conclusions
Osteolytic bone lesions and hypercalcemia as sole presentation of B-ALL in adults is extremely rare with only few cases reported worldwide. The prognostic implication of bone lesions and hypercalcemia in ALL is unclear and needs to be verified in large prospective studies. However, immediate recognition and treatment of hypercalcemia and the underlying B-ALL is vital since a delay of diagnosis poses a possible life-threatening risk.
| References | ▴Top |
- Kaiafa G, Perifanis V, Kakaletsis N, Chalvatzi K, Hatzitolios AI. Hypercalcemia and multiple osteolytic lesions in an adult patient with relapsed pre-B acute lymphoblastic leukemia: a case report. Hippokratia. 2015;19(1):78-81.
pubmed - Stein A, Boughton BJ. Common acute lymphoblastic leukaemia in an adult with hypercalcaemia and lytic bone lesions. Br J Haematol. 1988;70(4):503.
doi pubmed - Virijevic M, Vidovic A, Colovic N, Djunic I, Mitrovic M, Nada S-V, Tomin D. Hypercalcemia with multiple osteolytic lesions and increased circulating tumor necrosis factor in an adult patient with B-cell acute lymphoblastic leukemia. Srp Arh Celok Lek. 2016;144(3-4):207-210.
doi pubmed - Bassan R, Gatta G, Tondini C, Willemze R. Adult acute lymphoblastic leukaemia. Crit Rev Oncol Hematol. 2004;50(3):223-261.
doi pubmed - Verma SP, Dubashi B, Basu D, Dutta TK, Kar R. A rare case of adult acute lymphoblastic leukemia presenting with paraparesis and multiple osteolytic lesions. Indian J Hematol Blood Transfus. 2014;30(Suppl 1):24-26.
doi pubmed - Esbrit P. Hypercalcemia of malignancy—new insights into an old syndrome. Clin Lab. 2001;47(1-2):67-71.
pubmed - Shimonodan H, Nagayama J, Nagatoshi Y, Hatanaka M, Takada A, Iguchi H, Oda Y, et al. Acute lymphocytic leukemia in adolescence with multiple osteolytic lesions and hypercalcemia mediated by lymphoblast-producing parathyroid hormone-related peptide: a case report and review of the literature. Pediatr Blood Cancer. 2005;45(3):333-339.
doi pubmed - Granacher NCP, Berneman ZN, Schroyens W, Van de Velde ALR, Verlinden A, Gadisseur APA. Adult acute precursor B-cell lymphoblastic leukemia presenting as hypercalcemia and osteolytic bone lesions. Exp Hematol Oncol. 2017;6:9.
doi pubmed - Fukasawa H, Kato A, Fujigaki Y, Yonemura K, Furuya R, Hishida A. Hypercalcemia in a patient with B-cell acute lymphoblastic leukemia: a role of proinflammatory cytokine. Am J Med Sci. 2001;322(2):109-112.
doi pubmed - Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. World Health Organization Classification of Tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. IARC Press, Lyon, France. 2008. p. 150-155.
- Muller HL, Horwitz AE, Kuhl J. Acute lymphoblastic leukemia with severe skeletal involvement: a subset of childhood leukemia with a good prognosis. Pediatr Hematol Oncol. 1998;15(2):121-133.
doi pubmed - Lokadasan R, Prem S, Koshy SM, Jayasudha AV. Hypercalcaemia with disseminated osteolytic lesions: a rare presentation of childhood acute lymphoblastic leukaemia. Ecancermedicalscience. 2015;9:542.
doi pubmed - Siris ES, Grossbard L, Halper JP, Blume RS, Jacobs TP. Chronic lymphocytic leukemia and hypercalcemia: treatment with dichloromethylene diphosphonate. Metab Bone Dis Relat Res. 1983;5(2):61-63.
doi - Chung SW, Kim S, Choi JR, Yoo TH, Cha IH. Osteolytic mandible presenting as an initial manifestation of an adult acute lymphoblastic leukaemia. Int J Oral Maxillofac Surg. 2011;40(12):1438-1440.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


