| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 8, Number 2, June 2019, pages 83-85
Large Vessel Vasculitis as a Possible Mechanism of Vascular Side Effects of Ponatinib: A Case Report
Klaus Hirschbuehla, f, Andreas Ranka, Tim Pfeiffera, Tina Schallerb, Thomas Haeckelc, Joerg Roelingd, Dmytro Vlasenkoe, Martin Trepela, Christoph Schmida
aDepartment of Hematology and Oncology, Universitaetsklinikum Augsburg, Stenglinstrasse 2, 86156 Augsburg, Germany
bInstitute of Pathology, Universitaetsklinikum Augsburg, Stenglinstrasse 2, 86156 Augsburg, Germany
cDepartment of Radiology, Universitaetsklinikum Augsburg, Stenglinstrasse 2, 86156 Augsburg, Germany
dInternistische Gemeinschaftspraxis am Vincentinum, Franziskanergasse 14, 86152 Augsburg, Germany
eDepartment of Visceral Surgery, Universitaetsklinikum Augsburg, Stenglinstrasse 2, 86156 Augsburg, Germany
fCorresponding Author: Klaus Hirschbuehl, Department of Hematology and Oncology, Universitaetsklinikum Augsburg, Stenglinstrasse 2, 86156 Augsburg, Germany
Manuscript submitted May 13, 2019, accepted June 7, 2019
Short title: Ponatinib-Induced Vasculitis
doi: https://doi.org/10.14740/jh519
| Abstract | ▴Top |
Arterial occlusive events (AOEs) such as cerebrovascular, cardiovascular and peripheral arterial events are known side effects of ponatinib, assumed due to the rapid development and increase of arteriosclerosis, while the definitive pathomechanisms therefore are still unclear. We present a case of clinically apparent large vessel vasculitis and discuss this phenomenon as a possible mechanism of AOEs beside arteriosclerosis.
Keywords: Ponatinib; Arterial occlusive events; Vasculitis
| Introduction | ▴Top |
Tyrosine kinase inhibitors (TKIs) represent the standard treatment for chronic myeloid leukemia (CML) and, in addition to chemotherapy, Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). The third-generation TKI ponatinib has been shown to be effective in advanced diseases, including patients with T315I mutation and with relapse after allogeneic stem cell transplantation (alloSCT) [1-4]. However, arterial occlusive events (AOEs) such as cerebrovascular, cardiovascular and peripheral arterial events are known side effects of this drug, assumed due to the rapid development and increase of arteriosclerosis, while the definitive pathomechanisms therefore are still unclear [1, 5, 6]. We here present a case report of clinically apparent large vessel vasculitis and discuss this phenomenon also as a possible mechanism of AOEs beside arteriosclerosis.
| Case Report | ▴Top |
A 56-year-old woman underwent alloSCT for Ph+ ALL from a matched unrelated donor in March 2011 in first complete molecular remission (CMR). Due to molecular relapse 5 months from alloSCT, she was put on dasatinib and again achieved CMR. After hematological relapse 18 months from alloSCT and re-induction chemotherapy, a second alloSCT from a haplo-identical family donor was performed in November 2012. Again, CMR was achieved. Despite a transient chronic graft-versus-host disease of the skin, a second hematological relapse was diagnosed 2 years later, with detection of a T315I mutation within the bcr/abl fusion transcript. Therefore, the patient was put on ponatinib at a dose of 45 mg/day, upon which she responded rapidly and achieved again CMR. Due to increased liver enzymes (gamma glutamyltransferase 10 times the upper limit of normal range (ULN), alkaline phosphatase two times ULN) and dry, flaky skin, the ponatinib dose was reduced to 30 mg/day after 3 months of treatment, resulting in improvement of both side effects within 4 weeks. After 5 months on ponatinib, the patient developed rapidly increasing symptoms of intermittent claudication, with a final maximum walking distance of just a few meters. Extended vascular workup revealed long-segment stenosis of bilateral femoro-iliacal and subclavian arteries. Ultrasound showed a typical pattern for large vessel vasculitis with low-echo thickened vascular wall and no signs for arteriosclerosis (Fig. 1). After stop of ponatinib and initiation of therapy with steroids, claudication symptoms improved within 4 weeks. Within this time, ultrasound signs of vasculitis decreased as well. Due to a meningeal relapse of the ALL 16 months later, ponatinib was restarted in lack of available treatment alternatives. A dose of 15 mg/day was applied in combination with intrathecal chemotherapy (cytarabin, methotrexate and dexamethasone). This resulted in a CMR in the cerebrospinal fluid, but symptoms of intermittent claudication recurred. Therefore, steroids were administered again, while ponatinib was continued at 15 mg/day, resulting in slight improvement of leg pain and walking distance. Three months later, the patient was admitted to the emergency department with severe abdominal pain of acute onset. The computed tomography (CT) scan showed a distended cecum (7 cm) with no more clear delineation of the bowel wall, and a complete occlusion of the superior mesenteric artery (SMA; Fig. 2). The presence of collaterals suggested a chronic stenosis or occlusion of SMA. There were no signs of intestinal arteriosclerosis on the CT scan. Further, there was no evidence of atrial fibrillation in the patient’s history and the current electro-cardiogram that might have suggested a thromboembolic event. Immediate ileocecal surgical resection was performed. However, the patient died due to septic complications shortly thereafter. The histopathological evaluation of the resected compartment showed an infarction of the bowel wall but only minimal signs of both arteriosclerosis and inflammation.
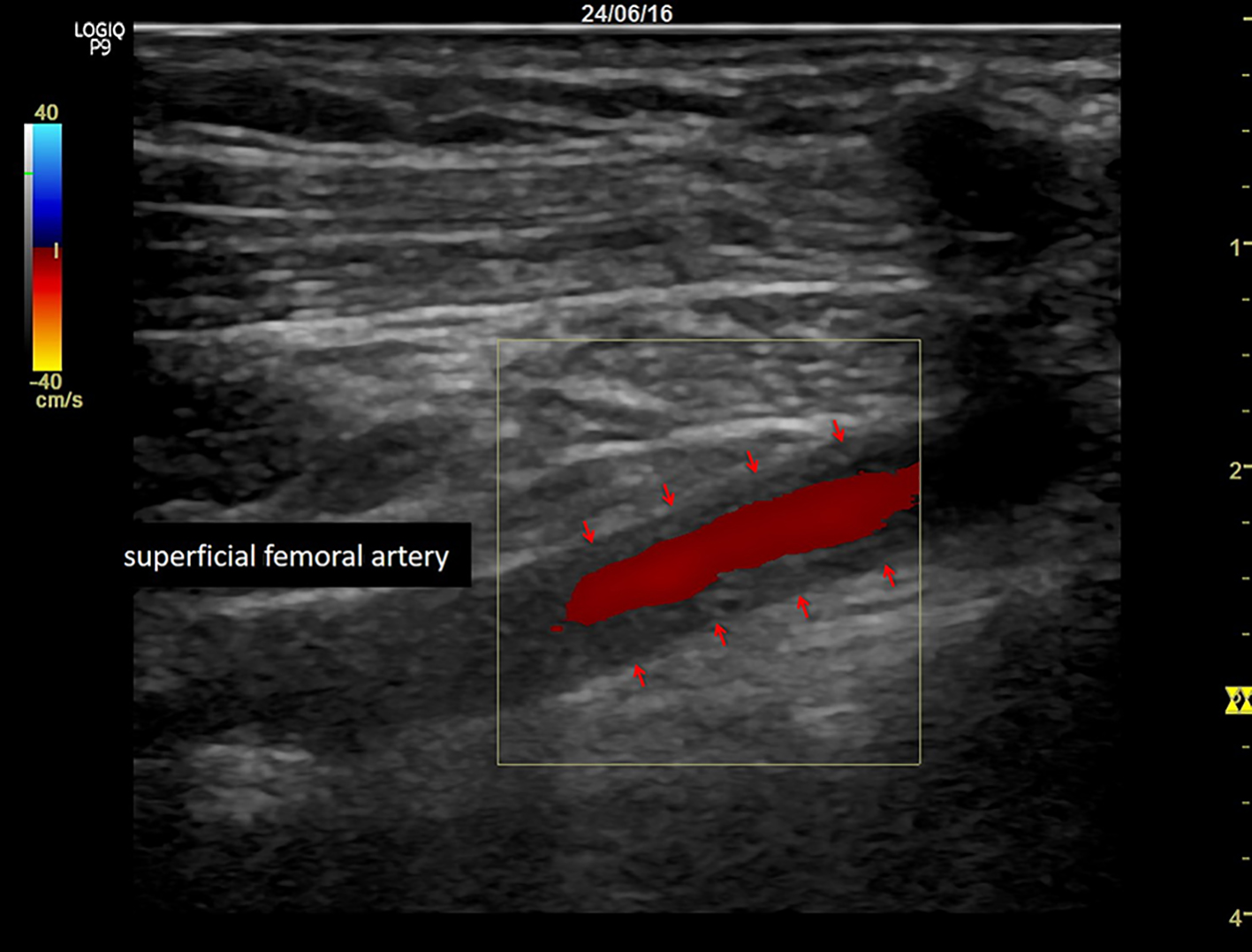 Click for large image | Figure 1. Typical pattern for large vessel vasculitis with low-echo thickened vascular wall (arrows). |
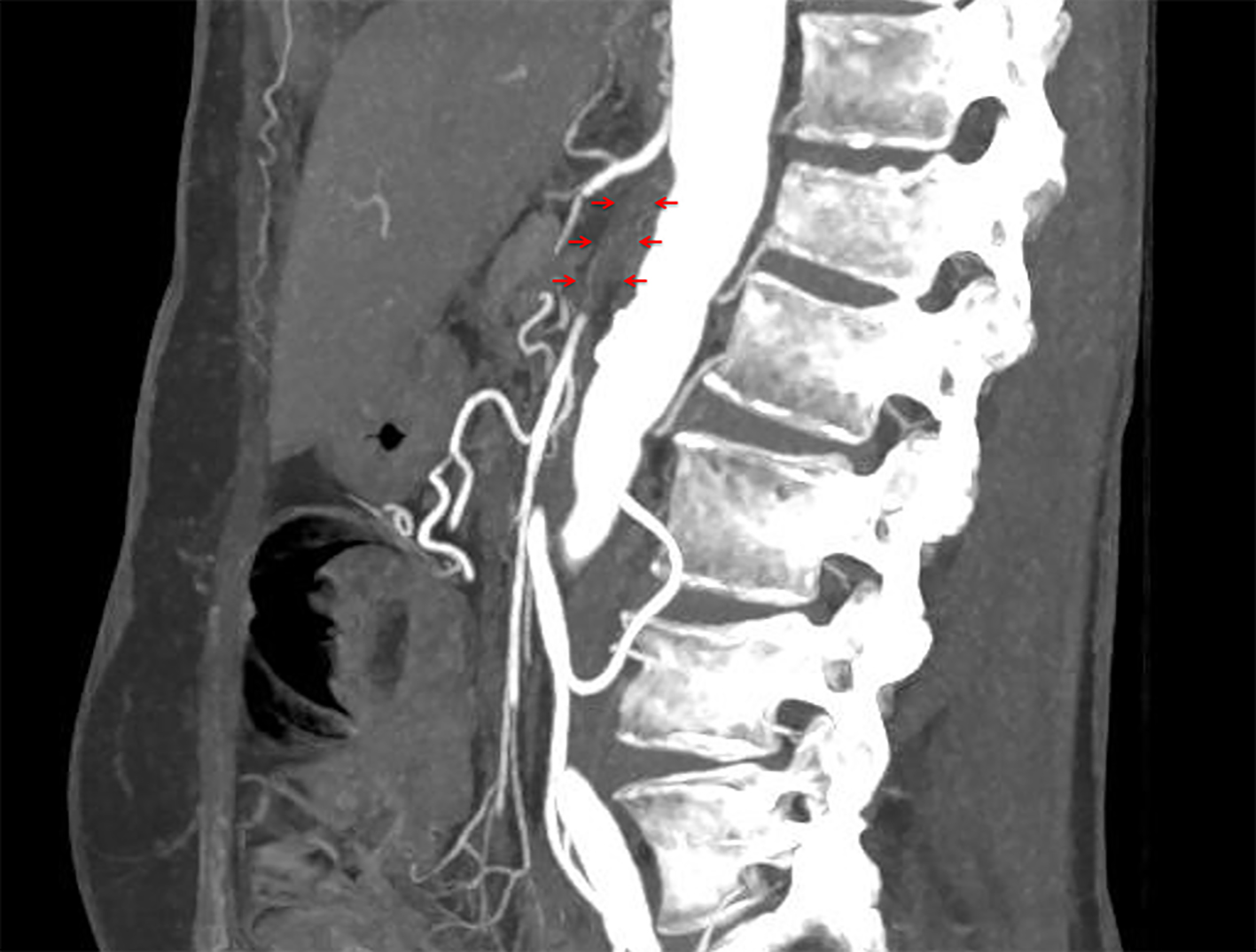 Click for large image | Figure 2. Occlusion of superior mesenteric artery (arrows). |
| Discussion | ▴Top |
Large vessel vasculitis as a possible mechanism for vascular events following ponatinib has been described in a patient with cerebral ischemia [7]. In accordance with this report, the present case suggests this pathomechanism by typical symptoms of claudication and ultrasound signs of vasculitis, as well as by rapid clinical improvement after cessation of ponatinib and steroid treatment. After re-exposition to ponatinib, claudication clinically re-appeared, and the patient finally died from an otherwise unexplained ischemic bowel disease. In the lack of other obvious reasons, intestinal vasculitis in the context of re-exposition to ponatinib was suspected as a trigger for the occlusion of the SMA. The missing clear proof of inflammation in histology in our case may be explained by the prior restart of steroids.
Pathogenesis of ponatinib-related vascular events might be mediated by vascular endothelial growth factor receptor 2 (VEGFR2) inhibition, leading to an increase in apoptosis as well as inhibition of viability and functional capacity of human umbilical vein endothelial cells (HUVECs) and endothelial progenitor cells (EPCs) in vitro [8, 9]. Further, exposition of HUVECs to serum of patients with thromboangiitis obliterans confers a diminished sprouting capacity [10]. Thus, one might speculate that there is a link between ponatinib, VEGFR2 inhibition and vasculitis.
Conclusion
As suggested previously [7], this case supports large vessel vasculitis as a possible mechanism for vascular complications of ponatinib beside arteriosclerosis. Therefore, we propose to consider vasculitis as a potential pathobiological factor in patients with perfusion deficits under ponatinib treatment. In addition, the drug should be used with great caution, once clinical signs of vasculitis are observed or pre-existent.
Acknowledgments
None.
Financial Disclosure
This work was not supported by any sponsorship (e.g. university, charity, commercial organization) or sources of material (e.g. novel drugs) not available commercially.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Klaus Hirschbuehl: writing manuscript. Andreas Rank: treating patient. Tim Pfeiffer: treating patient. Tina Schaller: doing histology. Thomas Haeckel: doing CT scan and providing image. Joerg Roeling: doing ultrasound and providing image. Dmytro Vlasenko: doing surgery. Martin Trepel: lector of the manuscript. Christoph Schmid: writing manuscript.
| References | ▴Top |
- Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, Nicolini FE, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132(4):393-404.
doi pubmed - Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, Nicolini FE, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783-1796.
doi pubmed - Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, O'Hare T, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367(22):2075-2088.
doi pubmed - Hirschbuehl K, Rank A, Pfeiffer T, Slawik HR, Schlimok G, Kolb HJ, Schmid C. Ponatinib given for advanced leukemia relapse after allo-SCT. Bone Marrow Transplant. 2015;50(4):599-600.
doi pubmed - Caldemeyer L, Dugan M, Edwards J, Akard L. Long-term side effects of tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Hematol Malig Rep. 2016;11(2):71-79.
doi pubmed - Valent P, Hadzijusufovic E, Schernthaner GH, Wolf D, Rea D, le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood. 2015;125(6):901-906.
doi pubmed - Mayer K, Gielen GH, Willinek W, Muller MC, Wolf D. Fatal progressive cerebral ischemia in CML under third-line treatment with ponatinib. Leukemia. 2014;28(4):976-977.
doi pubmed - Gover-Proaktor A, Granot G, Shapira S, Raz O, Pasvolsky O, Nagler A, Lev DL, et al. Ponatinib reduces viability, migration, and functionality of human endothelial cells. Leuk Lymphoma. 2017;58(6):1455-1467.
doi pubmed - Gover-Proaktor A, Granot G, Pasmanik-Chor M, Pasvolsky O, Shapira S, Raz O, Raanani P, et al. Bosutinib, dasatinib, imatinib, nilotinib, and ponatinib differentially affect the vascular molecular pathways and functionality of human endothelial cells. Leuk Lymphoma. 2019;60(1):189-199.
doi pubmed - Hewing B, Stangl V, Stangl K, Enke-Melzer K, Baumann G, Ludwig A. Circulating angiogenic factors in patients with thromboangiitis obliterans. PLoS One. 2012;7(4):e34717.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


