| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 8, Number 4, December 2019, pages 155-159
Superwarfarin Exposure: An Important Uncommon Cause of Painless Bleeding
Sreenath Kodalia, Tara Rajendrana, Ivan N. Richarda, Lakshmi Boyapatia, Edward C.C. Wongb, c, Boris Avezbakiyeva, d
aDivision of Hematology/Oncology, Brookdale University Hospital Medical Center, 1Brookdale Plaza, Brooklyn, NY 11212, USA
bQuest Diagnostics Nichols Institute, Chantilly, VA, USA
cGeorge Washington School of Medicine and Health Sciences, Departments of Pediatrics and Pathology, 2300 Eye Street, NW Washington DC, 20037, USA
dCorresponding Author: Boris Avezbakiyev, Division of Hematology/Oncology, Brookdale University Hospital Medical Center, 1Brookdale Plaza, Brooklyn, NY 11212, USA
Manuscript submitted July 4, 2019, accepted August 12, 2019
Short title: Superwarfarin Exposure Causes Painless Bleeding
doi: https://doi.org/10.14740/jh538
| Abstract | ▴Top |
Painless bleeding in a patient presenting from the community with elevated coagulation studies rarely makes the physicians suspect superwarfarin or rodenticide poisoning. Although a significant number of superwarfarin exposure cases are diagnosed every year, we believe there appears to be delay in diagnosis and confusion in determining what is the ideal way to treat and monitor these patients during the management. This is the first thorough literature review of all the reported cases of superwarfarin poisoning which also studied the clinical presentation, management and follow-up patterns. We present a 70-year-old man who presented to the emergency room with epistaxis, melena, cola-colored urine with elevated prothrombin time (PT), activated partial thromboplastin time (aPTT) and international normalized ratio (INR). Mixing studies showed complete correction of coagulopathy indicative of factor deficiency. Additional history revealed that the patient had arguments with family member at home and made us suspect superwarfarin exposure. Qualitative brodifacoum testing was positive and was managed with fresh frozen plasma and high doses of vitamin K1 (phytomenadione) with serial monitoring of INR and clinical symptoms. Superwarfarin poisoning should be considered in the differential diagnosis of a patient who presents with above clinical and laboratory profile especially in the absence of any history of coagulopathy or anticoagulant use. We want to raise public and especially physician awareness that history taking, early diagnosis and managing in right clinical setting play a significant role in survival of these patients.
Keywords: Superwarfarin; Phytomenadione; Painless bleeding
| Introduction | ▴Top |
This case report describes an uncommon cause of painless bleeding in an outpatient with markedly elevated international normalized ratio (INR) and normal liver function tests (LFTs). Superwarfarin poisoning should be considered in the differential diagnosis of a patient who presents with this clinical and laboratory profile in the absence of any history of coagulopathy or anticoagulant use.
| Case Report | ▴Top |
The patient was a 70-year-old man who presented to the emergency room with epistaxis, melena and cola-colored urine. He had first noticed the bleeding 5 days earlier, when he was blowing his nose and saw bright red blood on the tissue. It was not until he noticed black stool and cola-colored urine that he decided to come to the emergency room to be evaluated. His medical history included hypertension and type II diabetes mellitus. His medication history included daily baby aspirin and metformin, and a short course of azithromycin that was prescribed by his primary care provider for a suspected upper respiratory tract infection. The patient denied any family history of bleeding or thrombotic disorders and reported no personal history of smoking, alcohol, or illicit drug use. On physical examination, his vital signs were within normal limits: blood pressure (BP) 133/73 mm Hg, pulse rate (PR) 91, respiratory rate (RR) 24, temperature (T) 37.6 °C and peripheral capillary oxygen saturation (SpO2) 98% on room air. Physical examination showed gross bleeding from the right nostril. The lungs were clear to auscultation. The abdomen was soft without signs of trauma or bruising. Abnormal results of initial laboratory tests were as follows: hemoglobin, 10.5 (reference interval 13.4 - 15.4 g/dL); hematocrit (Hct), 32.4 (reference interval 40-47%); prothrombin time (PT) > 120 (reference interval 9.2 - 12.8 s); activated partial thromboplastin time (aPTT) > 240 (reference interval 23.5 - 33.5 s); INR > 10 (reference interval 0.7 - 1.2); and lactate dehydrogenase (LDH), 662 (reference interval 313 - 618 IU/L). Urinalysis (UA) was remarkable for 4+ blood and > 50 red blood cell/high power field (RBC/HPF). Computed tomography (CT) scans of the head and sinuses were unremarkable except for mucosal thickening in the right maxillary sinus. CT of the chest, abdomen and pelvis showed multilobar airspace infiltrates involving both lungs, most prominently in the left lower lobe, and a 2-cm right adrenal mass.
Because of the epistaxis and melena, the patient was admitted to the medical intensive care unit for close monitoring of supratherapeutic INR. Although a gastrointestinal consultation was requested, given the patient’s stable hemoglobin and Hct, urgent endoscopy was not indicated. Initial PT and aPTT mixing studies showed complete correction of coagulopathy indicative of factor deficiency (Table 1). The patient received four units of fresh frozen plasma (FFP) in the first 5 days of his hospital admission, which controlled his melena and hematuria and led to correction of his INR (Fig. 1). Concurrently, he was started on 10 mg vitamin K1 (phytomenadione) during the first 5 days of hospitalization (Fig. 2). Human immunodeficiency virus (HIV) and hepatitis panels were negative. Additional history revealed that the patient was the father of a 4-month-old baby with a new girlfriend, which led to arguments with his wife at home. He admitted that his current wife was cooking all his meals but did not suspect she would go to the extent of poisoning him. Despite not being on anticoagulants, some form of rodenticide or superwarfarin exposure was highly suspected, especially considering his extramarital affair and home situation. Although the possibility of any underlying malignancy (especially with CT of lung findings) was considered, given the lack of history of weight loss or smoking history and with the patient being up to date with health care screenings, this differential diagnosis was not pursued.
 Click to view | Table 1. Initial Screening and Follow-up Studies |
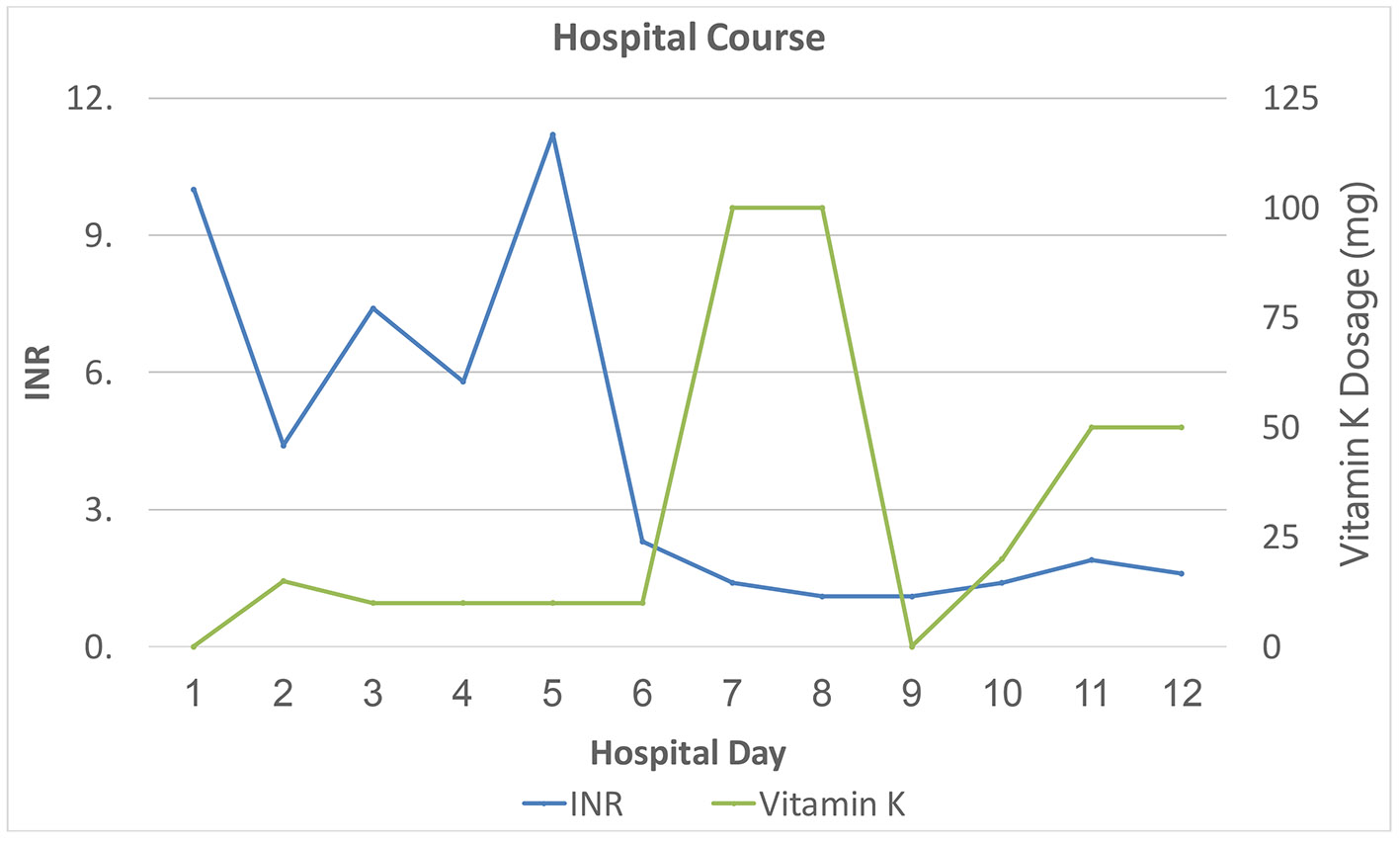 Click for large image | Figure 1. Inpatient course: INR in relation to treatment (plasma infusion: three units on the first day of hospitalization and one more unit on day 3). INR: international normalized ratio. |
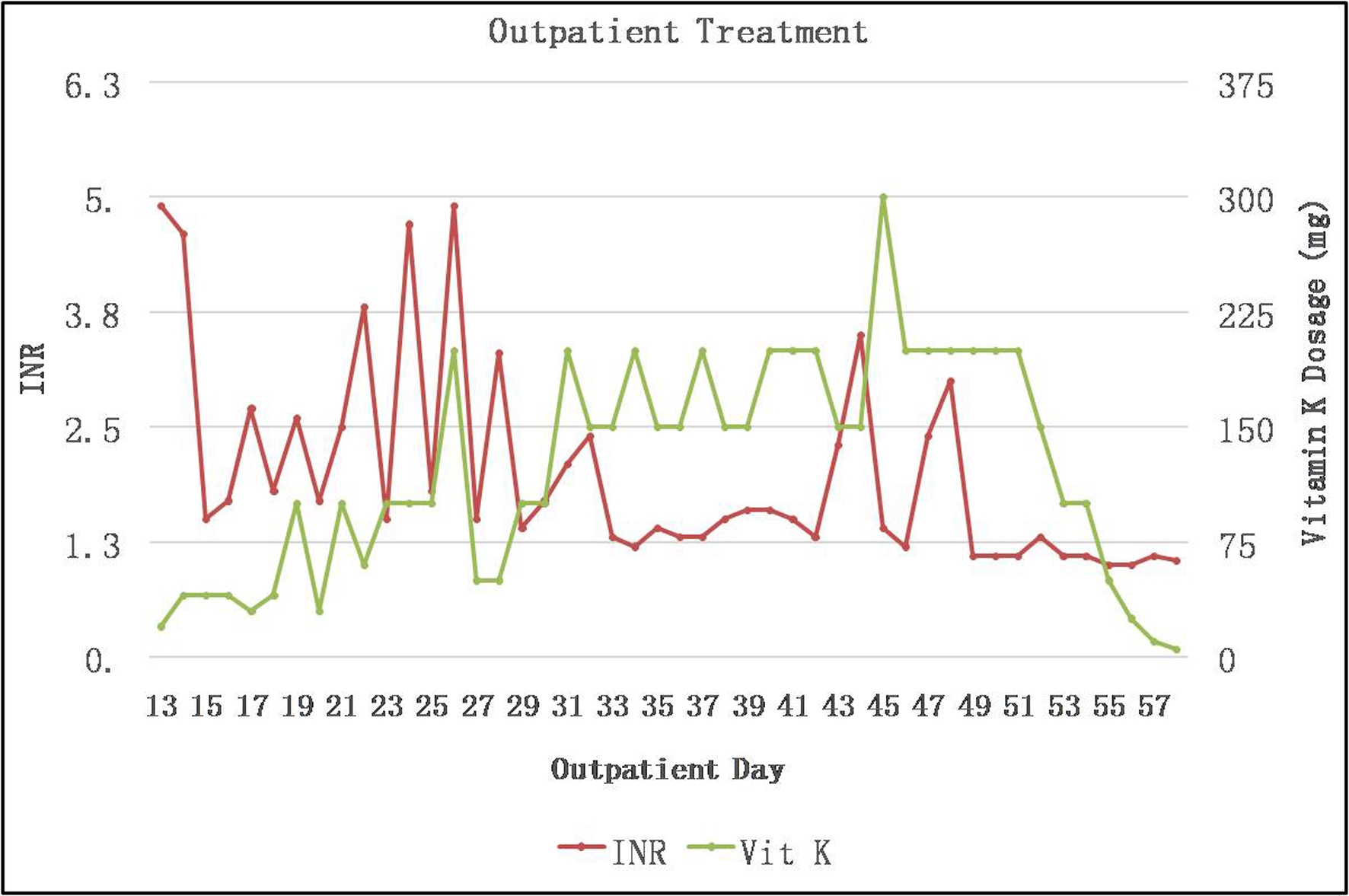 Click for large image | Figure 2. Outpatient course: INR in relation to treatment. INR: international normalized ratio. |
The local poison control center was notified and, based on their advice, blood specimens were sent for qualitative detection of brodifacoum. While awaiting the brodifacoum testing results, we also submitted specimens as noted in Table 1 for additional factor activity testing, antigen level measurement and autoimmune evaluation for acquired causes of coagulopathy. Based on the initial coagulation testing results and clinical history, the patient’s dose of vitamin K1 was increased to 100 mg daily on day 6, and he was eventually discharged on a dosage of 50 mg daily (Fig. 2). Brodifacoum testing eventually was reported to be positive using a high-performance liquid chromatography/tandem mass spectrometry (LC-MS/MS) assay (sensitive to 10 ng/mL). Factor antigens and activities were all normal except for factor VII and IX activity, which were markedly decreased. The results of all other autoimmune tests, including serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP), were negative. Because of a significant insurance copay issue in getting vitamin K1 as an outpatient, he was asked to follow up in our infusion unit daily initially, then every other day for high-dose oral vitamin K1 daily, followed by once a week, with treatment tapered over the next 5 months (Fig. 2). After an additional 3 months of monitoring, given his persistently normal INR and resolution of all symptoms, the decision was made to stop his vitamin K1 treatment.
| Discussion | ▴Top |
Warfarin was one of the first-generation vitamin K antagonists to be successfully used as anticoagulant rodenticide. Once rats developed resistance to it, second-generation anticoagulant rodenticides, called superwarfarins, were developed. These included brodifacoum, bromadiolone and difenacoum. These rodenticides are about 100 times more potent, and have 10 to 50 times longer half-life, with rapid onset of action and high lipid solubility compared to warfarin. Brodifacoum is principal ingredient in more than 90% of rodenticides in the USA [1]. As shown in Figure 3, these rodenticides strongly inhibit the vitamin K 2,3-epoxide reductase (VKOR), an enzyme that is essential for keeping vitamin K in the reduced, active hydroquinone form. Reduced vitamin K acts as a cofactor for the γ-carboxylation of vitamin K-dependent proteins by the enzyme gamma-glutamyl carboxylase. By inhibiting VKOR, gamma carboxylation of vitamin K-dependent clotting factors (factor II, VII, IX, X, protein C and S) is impaired.
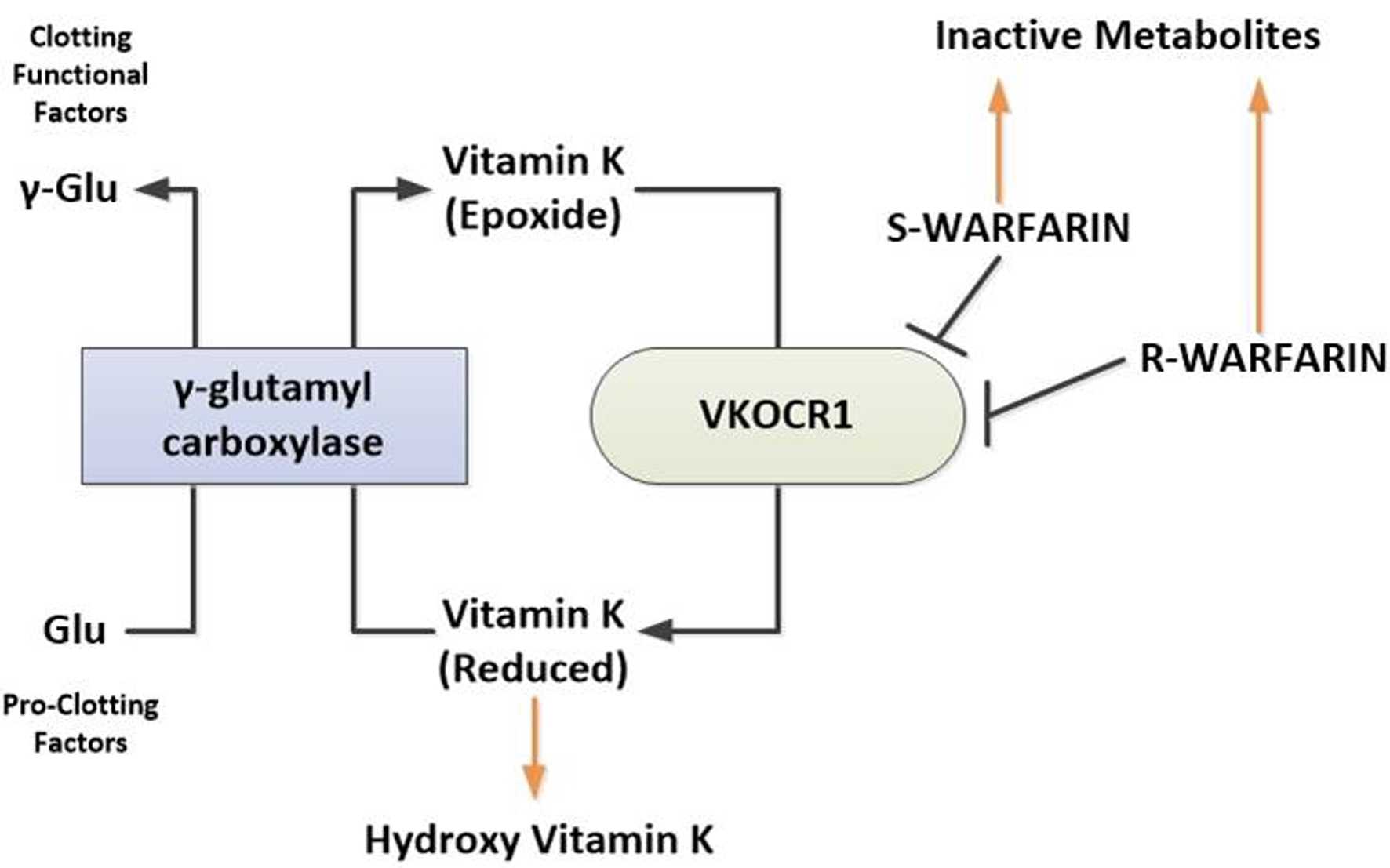 Click for large image | Figure 3. Mechanism of action of warfarin and superwarfarins. Brodifacoum and other superwarfarin similar to warfarin exert their effect by inhibiting VKOCR1. Inhibition of VKOCR1 leads to reduced bioavailability of the metabolically active reduced form of vitamin K resulting in decreased glutamyl carboxylation of vitamin K-dependent proteins including coagulation factors II, VII, IX and X, and protein S and C. S-warfarin and R-warfarin refer to S and R enantiomers of warfarin. VKORC1: vitamin K 2,3-epoxide reductase complex subunit 1. |
In adults, the usual route of superwarfarin toxicity is oral by intentional (suicidal vs. homicidal), or accidental (inhalation or by direct skin contact) exposure [2]. Most studies support first-order kinetic elimination for serum brodifacoum, with a half-life ranging from 16 to 36 days, but in rare cases, the brodifacoum half-life can be as long as 62 days. These differences in reported half-life values could be explained by patient-specific metabolism of brodifacoum. The metabolism of brodifacoum has not been delineated; however, since it has a substituted 4-hydroxycoumarin ring structure, its metabolism may be similar to warfarin. It is suspected to be hydroxylated to inactive compounds by mixed function oxidase enzymes in hepatic microsomes [3].
Signs and symptoms of superwarfarin poisoning include epistaxis and gingival bleeding, gastrointestinal and pulmonary hemorrhage, hemarthrosis and hematuria. The toxic dose of brodifacoum is difficult to determine, and a dose-response relationship has not been determined. Survivors usually have brodifacoum levels below 860 µg/L, but in the majority of cases both patients and clinicians do not know the brodifacoum concentration in the ingested product, and are unable to calculate ingested doses [4]. Although many forms of vitamin K exist, vitamin K1 (phytomenadione) is the strongest and longest acting, and is the preferred treatment in humans. Some studies recommend vitamin K every 6 h, as factor VII has a half-life of 3 - 5 h. However, daily dosing of vitamin K1 has been shown to lower PT and INR. The low frequency of dosing of vitamin K1 can be explained by the duration of action in steady-state conditions being near or possibly exceeding 24 h. Thus, some papers report an initial vitamin K1 dosage of 15 to 300 mg daily [5]. Currently, no standard treatment protocol exists for superwarfarin exposure. However, once superwarfarin exposure is suspected, the first step is to control the bleeding with packed red blood cells, FFP and vitamin K1 followed by daily vitamin K1. Patients with superwarfarin poisoning, if carefully monitored and treated, have a high survival rate.
Although much of the literature on this subject recommends following brodifacoum levels for months, we were unable to do so because of poor adherence to follow-up visits [6, 7]. Treatment endpoint in most case reports included discontinuation of vitamin K1 treatment while monitoring PT and aPTT [8]. Another proposed monitoring method is the evaluation of brodifacoum levels at regular intervals, with a goal of below 17 ng/mL or undetectable levels [9]. Another report recommended monitoring until the brodifacoum level is less than 10 µg/L [4]. However, it should be noted that only qualitative monitoring is available in the USA.
The relationship between bleeding and prolonged coagulation tests with long-acting anticoagulant rodenticide exposure is very well established and addressed in the literature [9]. Our case is especially important because it is the first time that extensive coagulation testing has been reported. Vitamin K-dependent factors were found to be abnormally low, while antigen levels were normal as note in Table 1. In the last 25 years, from 1992 to 2017, we found 16 published cases as detailed out in Supplementary Material 1 (www.thejh.org). Many of these case reports highlighted different clinical presentations and reported or evaluated different management approaches; however, none emphasized the importance of considering superwarfarin poisoning in the differential diagnosis in a patient presenting with painless bleeding. Furthermore, in these case reports, the day of presentation varied, likely depending on the amount ingested, age and co-morbidities of the patient. Among the 16 case reports reviewed, predominant clinical manifestations were epistaxis, hematuria and skin bruising, similar to our case. Other notable clinical manifestations included flank pain, bilateral loin pain, abdominal pain, suprapubic pain, chest pain, neck pain and headache. All cases were managed with vitamin K, which was tapered off slowly, and five (31%) of the 16 cases used FFP. As poor treatment adherence may lead to profuse re-bleeding [2] and our patient was non-adherent at home, we directly observed oral vitamin K1 ingestion in our outpatient clinic. We could not monitor quantitative levels of brodifacoum, as we believed our approach using basic coagulation screening and in-clinic verification of oral vitamin K1 treatment adequately treated the patient’s brodifacoum exposure. Given that the rodenticide exposure is still high with an incidence of over 8,000 cases in 2012, we hope that our case report in this clinical scenario will help to increase awareness of this public health issue [10, 11].
| Supplementary Material | ▴Top |
Supplementary Material 1. Meta Analysis of All the Reported Cases of Superwarfarin Poisoning.
Acknowledgments
We would like to thank Ms. Lina Noh for creation of the figures and tables.
Financial Disclosure
This report was supported by Brookdale Research Fund.
Conflict of Interest
None to declare.
Informed Consent
Patient’s informed consent was obtained.
Author Contributions
SK wrote the manuscript, reviewed the literature and took care of the patient. TR reviewed the literature and edited the manuscript. IR helped writing the manuscript and designing the tables and figures. LB helped editing the manuscript and reviewed literature. BA mentored to formulate ideas, gave suggestions to write manuscript and took care of the patient. ECCW helped with literature review, mentored to formulate ideas to write manuscript, edited the manuscript and helped ordering appropriate work up during the management of patient.
| References | ▴Top |
- World Health Organization. Brodifacoum health and safety guide. IPCS International Programme on chemical safety. Health and Safety Guide No. 93. http://www.inchem.org/documents/hsg/hsg/hsg093.htm. Accessed 2/14/2018.
- Lee HJ, You MR, Moon WR, Sul H, Chung CH, Park CY, Park SG. Evaluation of risk factors in patients with vitamin K-dependent coagulopathy presumed to be caused by exposure to brodifacoum. Korean J Intern Med. 2014;29(4):498-508.
doi pubmed - Jones EC, Growe GH, Naiman SC. Prolonged anticoagulation in rat poisoning. JAMA. 1984;252(21):3005-3007.
doi pubmed - Gunja N, Coggins A, Bidny S. Management of intentional superwarfarin poisoning with long-term vitamin K and brodifacoum levels. Clin Toxicol (Phila). 2011;49(5):385-390.
doi pubmed - Nelson AT, Hartzell JD, More K, Durning SJ. Ingestion of superwarfarin leading to co-agulopathy: a case report and review of the literature. MedGenMed. 2006;8(4):41.
- Chua JD, Friedenberg WR. Superwarfarin poisoning. Arch Intern Med. 1998;158(17):1929-1932.
doi pubmed - Binks S, Davies P. Case of the month: "Oh! Drat!—A case of transcutaneous superwarfa-rin poisoning and its recurrent presentation". Emerg Med J. 2007;24(4):307-308.
doi pubmed - Spahr JE, Maul JS, Rodgers GM. Superwarfarin poisoning: a report of two cases and re-view of the literature. Am J Hematol. 2007;82(7):656-660.
doi pubmed - Olmos V, Lopez CM. Brodifacoum poisoning with toxicokinetic data. Clin Toxicol (Phi-la). 2007;45(5):487-489.
doi pubmed - Feinstein DL, Brodsky S, Weinberg G, van Breeman R, Rubinstein I. Brodifacoum poi-soning: A clear and present danger to public health in the USA. Toxicol Lett. 2017;268:71-72.
doi pubmed - King N, Tran MH. Long-acting anticoagulant rodenticide (Superwarfarin) poisoning: a review of its historical development, epidemiology, and clinical management. Transfus Med Rev. 2015;29(4):250-258.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


