| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 9, Number 4, December 2020, pages 109-115
Reduce Mortality and Morbidity in Acute Myeloid Leukemia With Hyperleukocytosis With Early Admission in Intensive Care Unit: A Retrospective Analysis
Nathan Mottala, c, Nahema Issab, Pierre-Yves Dumasa, Fabrice Camoub, Mathieu Sauveziea, Francois-Xavier Grosa, Titouan Cazaubiela, Gaelle Mourissouxb, Harmony Leroya, Arnaud Pigneuxa, Olivier Guissetb, Thibaut Leguaya
aCHU de Bordeaux, Hopital Haut Leveque, Service d’Hematologie Clinique et Therapie Cellulaire, 1 Avenue de Magellan, 33600 Pessac, France
bCHU de Bordeaux, Hopital Saint Andre, Service de Reanimation medicale, 1 Rue Jean Burguet, 33000 Bordeaux, France
cCorresponding Author: Nathan Mottal, Service d’Hematologie Clinique et de Therapie Cellulaire, Centre Francois Magendie, Hopital Haut Leveque, 33604 Pessac, France
Manuscript submitted June 18, 2020, accepted August 4, 2020, published online October 1, 2020
Short title: Reduce Acute Myeloid Leukemia Mortality
doi: https://doi.org/10.14740/jh691
| Abstract | ▴Top |
Background: Patients presenting with acute myeloid leukemia (AML) at diagnosis are at high risk of severe complications and death, particularly with high white blood cell (WBC) count. In this retrospective study, we evaluate interest of early and systematic support in the intensive care unit (ICU) for AML with hyperleukocytosis (AML-HL) at diagnosis.
Methods: Patients with AML-HL, defined by WBC > 50 × 109/L, primary referred in ICU (“Early ICU”) without organ failure and before initiating chemotherapy induction were compared to patients first admitted in the Hematology Department who required a secondary transfer in ICU (“Late ICU”) or not (“No ICU”). Primary end point was mortality during the first month, and secondary end points were the use of life-sustaining therapies in ICU and risk factors for ICU transfer and mortality.
Results: One hundred fifty-four patients were included: 77 (50%) to the group “No ICU”, 18 (12%) to “Late ICU” and 59 (38%) to “Early ICU”. Mortality at day 30 was higher in “Late ICU” than in “Early ICU” and “No ICU”, with 27.8%; 16.9% and 2.6% respectively (P < 0.001). “Late ICU” patients had an increased use of life-sustaining therapy comparing to “Early ICU” patients (56% vs. 29%, P = 0.04).
Conclusions: Early referral to ICU reduces morbidity and seems an effective strategy to reduce short-term mortality in AML-HL at diagnosis.
Keywords: Acute myeloid leukemia; Hyperleukocytosis; Intensive care unit; Mortality
| Introduction | ▴Top |
Patients presenting with acute myeloid leukemia (AML) at diagnosis are at high risk of severe complications and death. Clinical presentation of AML is variable. It may be led by hemorrhages induced by disseminated intravascular coagulation (DIC) and thrombocytopenia; or leukostasis, which is responsible for cerebral or pulmonary impairment. Tumor lysis syndrome (TLS) is the main cause of renal impairment. TLS and infections may be present upon diagnosis but are much favored by induction chemotherapy. Furthermore, specifics organ toxicities of chemotherapy and prolonged pancytopenia due to induction therapy strongly burden the prognosis.
Early complications, related to leukemia, are mainly based on AML subtype and white blood cells (WBC) count, with an increased risk of mortality in patients with subtype 3; 4 or 5 (French-American-British (FAB) classification) and/or hyperleukocytosis (HL) [1, 2] and/or DIC.
Referring patients with AML in intensive care unit (ICU) for these complications is frequent. Recent evidences show that ICU admission for these critically ill patients is benefit in terms of mortality [3]. Despite an increased risk of infection, mainly due to mechanical ventilation, non-invasive therapeutic strategies used in ICU contributed to improved overall survival [4, 5]. Patients who survive complications and ICU recover with complete remission rate and global survival rate of their cytogenetic subgroup.
However, prognosis of patients with AML in ICU remains poor with 41-61% of in-ICU mortality reviewed in the past decade [6-11]. Strategies have risen to improve outcome, especially concerning the timing of admission. ICU transfer is associated with increased mortality but early admissions seem beneficial comparing with late admissions [12]. Late admission is associated with non-invasive ventilation failure and similarly, in patients requiring mechanical ventilation, a late intubation is associated with a higher mortality [13, 14].
In this retrospective study, we evaluate interest of early support in ICU in terms of morbidity (use of life-sustaining therapy (LST)) and mortality in AML with HL. The secondary purpose was to highlight the risk factors of secondary transfer in ICU.
| Materials and Methods | ▴Top |
The study was approved by the Institutional Review Board (CNIL registration number: DR-2015-529). The design of the study complies with the GDPR and the framework set by article 65-2 of the French Data Protection Act 2018 as amended.
We retrospectively reviewed all AML with HL, defined by WBC > 50 × 109/L, diagnosed in Bordeaux Hospital between 2008 and 2016. Patients were included in the current study if they were ≥18 years of age, treated by intensive chemotherapy for at least 1 day and had received no previous treatment for AML except for hydroxycarbamide. Patients admitted in ICU for an organ failure (except hematological failure) prior to the first day of chemotherapy were excluded. Written informed consent was obtained in accordance with the Declaration of Helsinki, allowing for the collection of clinical data in the anonymized database.
Until 2012, patients with AML-HL at diagnosis were referred in the Hematology Department to initiate chemotherapy induction. This group of patients has been allocated into two subgroups, depending on necessity of a secondary transfer in ICU for complication during induction (“Late ICU”) or not (“No ICU”). Decision to transfer a patient to ICU was always made by both hematologists and intensivists. Patients were transferred back to the Hematology Department when medical condition allowed it. In 2012, there was a change in medical strategy in our department, and it has been decided that patients with AML-HL would be primary referred in ICU for the diagnostic workup and to initiate induction chemotherapy. They were transferred later in Hematology Department for the following support. This group of patients is designated as “Early ICU”. Patients could be transferred back in ICU in case of need. In each group, patients could carry on chemotherapy in ICU. Management of patients in ICU was always made with daily close collaboration between hematologists and intensivists.
Date of diagnosis was considered as the date of bone marrow aspiration. Biological values were those of the day of diagnosis. Biological DIC was defined as an increased of plasmatic D-dimer with a prothrombin time below 50% or platelets below 50 × 109/L. Clinical parameters (leukostasis, performance status (PS), clinical DIC) were also collected the day of diagnosis. The use of cytoreductive therapy was at the discretion of clinicians in the Hematology Department. All patients in the group “Early ICU” had cytoreductive therapy by dexamethasone and hydroxyurea (HU) upon admission. Response criteria were defined according to the current international recommendations. Induction chemotherapy consisted for all patients in a classic “3 + 7” regimen containing standard dose of cytarabine and anthracycline. Some of them had an addition of lomustine, according to the current clinical trials. Response criteria were defined according to international recommendations [15].
The primary purpose of this study was to evaluate the interest of early admission in ICU in terms of mortality and morbidity during induction phase. Mortality was assessed on the seventh and the 30th day after the onset of induction in the three groups. Morbidity was assessed between the groups “Early ICU” and “Late ICU” during the first month of treatment by the length of stay and the use of LST. LSTs were defined by use of vasopressors agents; renal replacement therapy (RRT) or invasive and non-invasive mechanical ventilation (i-MV and NIV). The number of ventilation-free days (VFD) was calculated for each patient who has stayed in ICU and defined as one point each day that patients are both alive and free from i-MV. The secondary purpose was to assess risk factors of ICU transfer and mortality.
Statistical analysis
We described the patients’ characteristics at diagnosis using numbers and frequencies for qualitative data, and the median, interquartile range (IQR), and range (minimum - maximum) for quantitative data. For survival analyses, Kaplan-Meier survival curves were drawn
| Results | ▴Top |
Patients’ characteristics
Between January 2008 and December 2016, 203 patients were admitted in our center for AML-HL and received at least 1 day of chemotherapy induction. Thirteen were excluded for being admitted in ICU before chemotherapy for an organ failure and 36 were excluded for being dead before initiating chemotherapy or for being ineligible for intensive chemotherapy. Data from the remaining 154 patients (83 men and 71 women) were analyzed. Fifty-nine patients have been referred in ICU before initiating the treatment and 95 have been referred in the Hematology Department. Among these 95 patients, 18 needed to be transferred in ICU during induction phase and 77 had never been in ICU (Fig. 1).
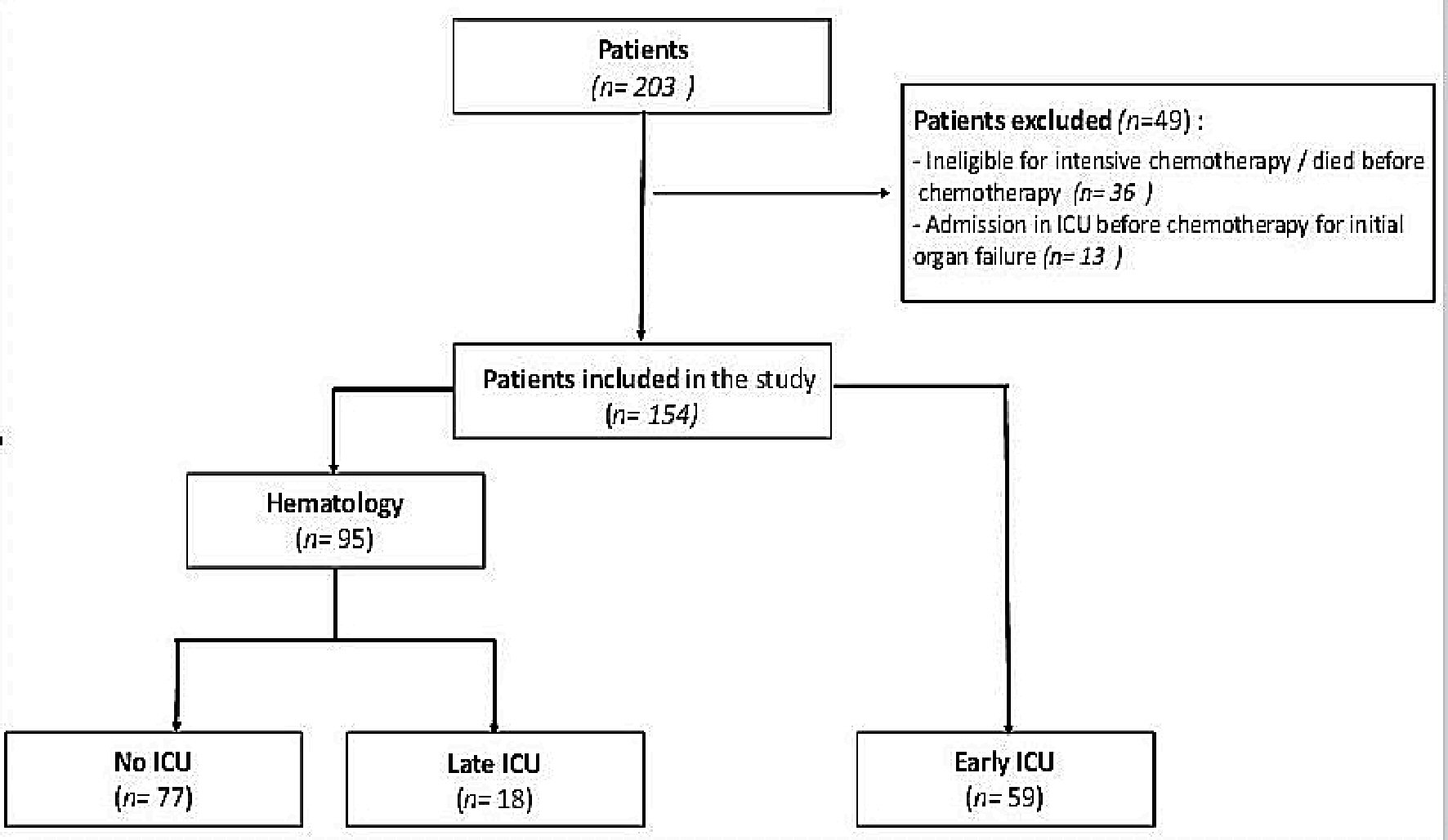 Click for large image | Figure 1. Flow charts. ICU: intensive care unit. |
The median age was 60 years (range: 17 - 79; IQR: 46 - 69). Eighty-four (54%) had an AML subtype 4 or 5 according to FAB classification. The median PS was 1 and median Charlson comorbidity index was 0. The median initial WBC was 92 × 109/L (range: 50 - 457; IQR: 66 - 149). Twenty-six patients (16.9%) had pulmonary or cerebral leukostasis and the rate of biological and clinical DIC was 18.8% and 3.2% respectively. Characteristics are shown in Table 1.
 Click to view | Table 1. Characteristics and Outcomes |
There was no difference between the three groups in terms of age, PS or comorbidity (assessed by modified Charlson comorbidity index). There was a significant difference between the three groups in terms of WBC with 74 × 109/L (IQR: 61 - 104) in the “No ICU” patients, 68 × 109/L (IQR: 56 - 155) in the “Late ICU” patients and 140 × 109/L (IQR: 91 - 197) in the “Early ICU” patients (P < 0.001). There also was a significant difference in the rate of leukostasis (6%, 33% and 25%, respectively, P < 0.001) and biological DIC (12%, 39% and 22%, respectively, P = 0.025). The rate of complete remission was 78% for “No ICU” patients, 61% for “Late ICU” patients and 68% for “Early ICU” patients (P = 0.23).
In the “Late ICU” group, median time for ICU admission was the sixth day after initiating chemotherapy induction (IQR: 3 - 13).
Mortality
The 30-day mortality rate in the entire cohort was 11% (n = 17) with 2.6% (n = 2) in the “No ICU” patients; 27.8% (n = 5) in the “Late ICU” patients and 16.9% (n = 10) in the “Early ICU” patients (P < 0.001) (Fig. 2). The 7-day mortality rate in the all cohort was 6.5% (n = 10) with 1.3% (n = 1); 16.7% (n = 3) and 10.2% (n = 6) in the three groups, respectively (P = 0.008).
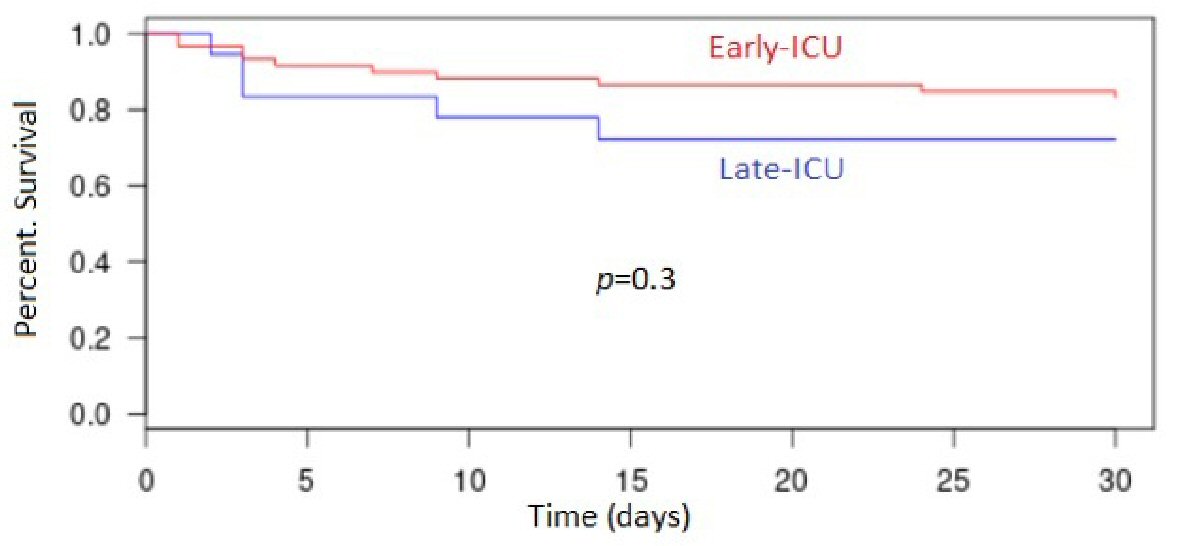 Click for large image | Figure 2. Kaplan-Meyer estimates of overall survival in groups “Early ICU” and “Late ICU”. ICU: intensive care unit. |
Of the 10 deaths occurring in the first 7 days, five resulted from DIC with intracerebral hemorrhage (ICH), three from TLS, and two from unknown cause. Of the seven deaths occurring later, four resulted from infection including one from pneumocystosis, one from DIC with ICH, and two from heart failure. In the “Early ICU” patients, causes of death were DIC with ICH (n = 4), septic shock (n = 3), heart failure (n = 2) and TLS (n = 1).
Morbidity
Concerning severity of the ICU stay, the “Late ICU” patients had an increased use of LST comparing to “Early ICU” patients (56% vs. 29%, P = 0.04). They had a higher need of EER (11% vs. 5%, P = 0.33), amines (33 vs. 12%, P = 0.07) and i-MV (44% vs. 19%, P = 0.06). Also, VFD was lower in the “Late ICU” group with 90 cumulative days for 157 cumulative days of stay in ICU versus 246 cumulative days for 260 cumulative days in ICU in the group “Early ICU” (P < 0.001)
The median length of stay in ICU was not significantly different with 3 days (IQR: 2 - 7) in “Late ICU” patients and 4 days (IQR: 2 - 6) in “Early ICU” patients (P = 0.81).
In the group “Early ICU”, the rate of secondary transfer in ICU was 11.9% (n = 7). In the 95 patients in the “Hematology” group, the rate of ICU transfer was 20% (n = 19, one “Late ICU” patient was transferred twice) (P = 0.38).
Concerning infections, possible and probable invasive fungal diseases were seen respectively in three and four cases in “No ICU” patients, one and two cases in “Late ICU” patients and zero and three cases in “Early ICU” patients. All cases were awarded to Aspergillus sp. but one to Candida sp. The three patients in “Late ICU” group with fungal disease were diagnosed after their return to the Hematology Department. Septic shock occurred in six patients (33.3%) in “Late ICU”, and seven (11.9%) in “Early ICU”.
Risk factors of ICU transfer and mortality
In the 157 patients, characteristics significantly associated with mortality in univariate analysis were PS ≥ 2 (P = 0.034); clinical DIC (P = 0.015) and AML subtype 4 or 5 (P = 0.036). In multivariate analysis, AML subtype was the only independent risk factor of mortality (P = 0.041).
In the 96 patients of “Hematology” group, risk factors of ICU transfer in univariate analysis were modified Charlson score > 1 (P = 0.008); C-reactive protein (CRP) > 54 mg/L (P = 0.05); leukostasis (P < 0.001) and biological DIC (P = 0.003). In multivariate analysis, leukostasis and biological DIC stayed independent prognostic factors of ICU transfer during induction (P = 0.003 and P = 0.029, respectively).
| Discussion | ▴Top |
Close collaboration between intensivists and hematologists is essential in management of patients with AML during induction therapy. It is obvious that the intensivists taking care of patients with AML must be in the habits of managing chemotherapy and specific complications of both leukemia and chemotherapy. Strategy of early admission in ICU has been retrospectively studied for high-risk AML and seems beneficial comparing with late admission [3].
Our study suggests a benefit in term of mortality with this early-ICU strategy but we did not succeed in highlighting it significantly. The number of patients is probably insufficient and responsible for the lack of power. One of the main reasons of the insignificant results may be the differences in the groups in WBC count and leukostasis, which were significantly higher in the group “Early ICU”. This study was aimed to compare two strategies of care in patients with AML-HL at diagnosis, each used at a different time in our center. Indeed, most patients before 2012 were primary admitted in the Hematology Department for chemotherapy induction but the admission in Hematology or ICU was a clinician decision. Clinicians could be influenced by factors of severity such WBC > 100 × 109/L or clinical leukostasis. In the current study, four patients were primary admitted in ICU before 2012. They all had WBC count > 100 × 109/L and one of them presented with pulmonary leukostasis. Conversely, clinicians could be reassured by WBC < 100 × 109/L, the lack of leukostasis or DIC and decided to treat patient in the Hematology Department. Indeed, of the 95 patients in the group “Hematology”, 50 of them had diagnosis after 2012. Only seven of them had WBC > 100 × 109/L, four of them had clinical leukostasis and only one died within the 30th day. This practice at the discretion of the clinicians may explain here the differences in the characteristics of the groups.
According to what we expected, most deaths occurred precociously (in the first 7 days), and were directly linked to leukemia. The median time for ICU admission in the “Late ICU” group was 6 days, which means that severe complications during induction are more often related to leukemia than to chemotherapy or aplasia in patient with AML and WBC > 50 × 109/L. These results suggest a clear benefit for these high-risk patients to be in ICU during induction therapy.
In regard to morbidity, “Late ICU” patients had more complications during ICU stay comparing with “Early ICU” patients. We observed a significant increased use of i-MV and lower number of VFD, despite no difference in median length of stay. The use of NIV may explain this difference by an earlier care of any respiratory distress. Conversely, patients in the Hematology Department may spend a few days with a growing need for oxygen before being transferred in ICU with a more advanced respiratory failure. In literature, the early use of NIV has showed to improve survival in immune-compromised patients with acute respiratory failure [16].
About cytoreductive therapy, there is no standardized procedure in our center. The use of HU or dexamethasone was at the discretion of clinicians in the Hematology Department and was systematic at ICU admission, resulting in statistical difference between the three groups. However, WBC count at diagnosis was 74 × 109/L in “No ICU” group, 68 × 109/L in “Late ICU” and 140 × 109/L in “Early ICU” while, at day 1 of chemotherapy induction, it had decreased to 59 × 109/L, 65 × 109/L and 118 × 109/L. This might reflect the benefit of the cytoreductive strategy in some patients. Despite cytoreductive strategy with HU is recommended in AML-HL by recent international guidelines, it is obvious that it must never delay intensive chemotherapy when patients are eligible [15, 17]. Otherwise, data about cytoreductive therapy by HU or leukapheresis remain controversial [18], and a recent study failed to find any correlation between HU sensitivity and short- or long-term outcome in AML-HL [19].
One hundred percent of patients in the group “Early ICU” received dexamethasone before initiating chemotherapy and during the first days of chemotherapy induction. These patients had a higher WBC count, which results in a severe inflammatory status and frequent leukostasis syndrome. Myeloblasts have indeed the ability to secrete adhesion receptors and cytokines, leading to tissue infiltration and leukostasis [20]. Because of its inhibitor effect on cytokines production, dexamethasone is widely used in ICU in most critically ill AML patients. In recent literature, adding dexamethasone to conventional chemotherapy showed a significant impact on relapse incidence and overall survival, but no benefit on early mortality [21]. A retrospective study suggests a benefit of adding dexamethasone, in term of MV requirement and ICU mortality in monoblastic AML with specific lung injury [22].
Conclusions
Early referral to ICU seems an effective strategy to reduce early mortality for AML-HL at diagnosis, but it has to be confirmed in a larger cohort. We demonstrate here that late admission in ICU is associated with drastically increase and longer use of life-sustaining therapy.
Acknowledgments
None to declare.
Financial Disclosure
There are no financial conflicts of interest to disclose.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Written informed consent was obtained.
Author Contributions
Nathan Mottal collected the data, analyzed the data and wrote the manuscript. Nahema Issa contributed to the analysis of the results and writing of the manuscript. Pierre-Yves Dumas contributed to the analysis of the results, writing of the manuscript and the design of the project. Fabrice Camou designed the project, and contributed to the analysis of the results and writing of the manuscript. Mathieu Sauvezie analyzed the data, and contributed to the analysis of the results. Francois-Xavier Gros contributed to the writing of the manuscript. Titouan Cazaubiel contributed to collect the data. Gaelle Mourissoux supervised the work. Harmony Leroy contributed to the writing of the manuscript. Arnaud Pigneux supervised the work, and contributed to the writing of the manuscript. Olivier Guisset supervised the work. Thibaut Leguay designed and directed the project, and contributed to the analysis of the results and the writing of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Porcu P, Cripe LD, Ng EW, Bhatia S, Danielson CM, Orazi A, McCarthy LJ. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leuk Lymphoma. 2000;39(1-2):1-18.
doi pubmed - Wong GC. Hyperleukocytosis in acute myeloid leukemia patients is associated with high 30-day mortality which is not improved with leukapheresis. Ann Hematol. 2015;94(12):2067-2068.
doi pubmed - Lengline E, Raffoux E, Lemiale V, Darmon M, Canet E, Boissel N, Schlemmer B, et al. Intensive care unit management of patients with newly diagnosed acute myeloid leukemia with no organ failure. Leuk Lymphoma. 2012;53(7):1352-1359.
doi pubmed - Azoulay E, Alberti C, Bornstain C, Leleu G, Moreau D, Recher C, Chevret S, et al. Improved survival in cancer patients requiring mechanical ventilatory support: impact of noninvasive mechanical ventilatory support. Crit Care Med. 2001;29(3):519-525.
doi pubmed - Razlaf P, Pabst D, Mohr M, Kessler T, Wiewrodt R, Stelljes M, Reinecke H, et al. Non-invasive ventilation in immunosuppressed patients with pneumonia and extrapulmonary sepsis. Respir Med. 2012;106(11):1509-1516.
doi pubmed - Roze des Ordons AL, Chan K, Mirza I, Townsend DR, Bagshaw SM. Clinical characteristics and outcomes of patients with acute myelogenous leukemia admitted to intensive care: a case-control study. BMC Cancer. 2010;10:516.
doi pubmed - Ahmed T, Koch AL, Isom S, Klepin HD, Bishop JM, Ellis LR, Berenzon D, et al. Outcomes and changes in code status of patients with acute myeloid leukemia undergoing induction chemotherapy who were transferred to the intensive care unit. Leuk Res. 2017;62:51-55.
doi pubmed - Halpern AB, Culakova E, Walter RB, Lyman GH. Risk factors, mortality, and care costs of adults with acute myeloid leukemia admitted to the intensive care unit: a longitudinal cohort study. JAMA Oncol. 2017;3:374-381.
doi pubmed - Jackson K, Mollee P, Morris K, Butler J, Jackson D, Kruger P, Klein K, et al. Outcomes and prognostic factors for patients with acute myeloid leukemia admitted to the intensive care unit. Leuk Lymphoma. 2014;55(1):97-104.
doi pubmed - Sippel C, Kim Y, Wallau A, Brossart P, Schmidt-Wolf I, Walger P. AML versus ICU: outcome of septic AML patients in an intensive care setting. J Cancer Res Clin Oncol. 2015;141(9):1645-1651.
doi pubmed - Schellongowski P, Staudinger T, Kundi M, Laczika K, Locker GJ, Bojic A, Robak O, et al. Prognostic factors for intensive care unit admission, intensive care outcome, and post-intensive care survival in patients with de novo acute myeloid leukemia: a single center experience. Haematologica. 2011;96(2):231-237.
doi pubmed - Mokart D, Lambert J, Schnell D, Fouche L, Rabbat A, Kouatchet A, Lemiale V, et al. Delayed intensive care unit admission is associated with increased mortality in patients with cancer with acute respiratory failure. Leuk Lymphoma. 2013;54(8):1724-1729.
doi pubmed - Adda M, Coquet I, Darmon M, Thiery G, Schlemmer B, Azoulay E. Predictors of noninvasive ventilation failure in patients with hematologic malignancy and acute respiratory failure. Crit Care Med. 2008;36(10):2766-2772.
doi pubmed - Depuydt PO, Benoit DD, Vandewoude KH, Decruyenaere JM, Colardyn FA. Outcome in noninvasively and invasively ventilated hematologic patients with acute respiratory failure. Chest. 2004;126(4):1299-1306.
doi pubmed - Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447.
doi pubmed - Huang HB, Xu B, Liu GY, Lin JD, Du B. Use of noninvasive ventilation in immunocompromised patients with acute respiratory failure: a systematic review and meta-analysis. Crit Care. 2017;21(1):4.
doi pubmed - Rollig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood. 2015;125(21):3246-3252.
doi pubmed - Oberoi S, Lehrnbecher T, Phillips B, Hitzler J, Ethier MC, Beyene J, Sung L. Leukapheresis and low-dose chemotherapy do not reduce early mortality in acute myeloid leukemia hyperleukocytosis: a systematic review and meta-analysis. Leuk Res. 2014;38(4):460-468.
doi pubmed - Cerrano M, Seegers V, Raffoux E, Rabian F, Sebert M, Itzykson R, Lemiale V, et al. Predictors and outcomes associated with hydroxyurea sensitivity in acute myeloid leukemia patients with high hyperleukocytosis. Leuk Lymphoma. 2020;61(3):737-740.
doi pubmed - Stucki A, Rivier AS, Gikic M, Monai N, Schapira M, Spertini O. Endothelial cell activation by myeloblasts: molecular mechanisms of leukostasis and leukemic cell dissemination. Blood. 2001;97(7):2121-2129.
doi pubmed - Bertoli S, Picard M, Berard E, Griessinger E, Larrue C, Mouchel PL, Vergez F, et al. Dexamethasone in hyperleukocytic acute myeloid leukemia. Haematologica. 2018;103(6):988-998.
doi pubmed - Azoulay E, Canet E, Raffoux E, Lengline E, Lemiale V, Vincent F, de Labarthe A, et al. Dexamethasone in patients with acute lung injury from acute monocytic leukaemia. Eur Respir J. 2012;39(3):648-653.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


