| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Review
Volume 9, Number 4, December 2020, pages 97-108
The Impact of Preoperative Intravenous Iron Therapy on Perioperative Outcomes in Cardiac Surgery: A Systematic Review
Kelly A. Tankarda, Brian Parka, Ethan Y. Brovmanb, Angela M. Badera, c, Richard D. Urmana, c, d
aDepartment of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis St, Boston, MA 02115, USA
bDivision of Cardiac Anesthesiology, Department of Anesthesiology and Perioperative Medicine, Tufts Medical Center, Tufts University School of Medicine, 800 Washington St, Boston, MA 02111, USA
cCenter for Surgery and Public Health, Brigham and Women’s Hospital, Boston, MA 02115, USA
dCorresponding Author: Richard D. Urman, Department of Anesthesiology, Perioperative and Pain Medicine, Center for Perioperative Research, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA
Manuscript submitted June 7, 2020, accepted August 18, 2020, published online October 1, 2020
Short title: Preoperative IV Iron in Cardiac Surgery
doi: https://doi.org/10.14740/jh696
| Abstract | ▴Top |
Background: Anemia is common in cardiac surgery affecting 25-40% of patients and associated with increased blood transfusions, morbidity, mortality, and higher hospital costs. Higher rates of stroke, acute renal injury, and total number of adverse postoperative outcomes have also been reported to be associated with preoperative anemia. This systematic review assessed the current evidence for preoperative intravenous iron on major outcomes following cardiac surgery.
Methods: Outcome measures included postoperative hemoglobin, transfusion rates, major adverse events, and mortality. The review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and articles were identified using PubMed, Cochrane, CLINAHL, WOS, and EMBASE databases. Articles were included if they compared patients with and without preoperative anemia based on treatment with intravenous iron. Quality was assessed using Cochrane Risk of Bias Tool and Newcastle-Ottawa scale, and strength of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach.
Results: Of the articles reviewed, six met inclusion criteria. These included four randomized double-blind prospective cohort studies, one randomized non-blinded prospective study, and one non-randomized non-blinded prospective study with historical control. Across studies, 1,038 patients were enrolled. Two studies showed higher hemoglobin with iron therapy, and only one study showed significant differences in multiple outcomes such as transfusion and morbidity.
Conclusions: Given the paucity of studies and biases within them, the current evidence for treatment with intravenous iron prior to cardiac surgery is weak. More evidence is needed to support the administration of preoperative intravenous iron in cardiac surgery patients.
Keywords: Anemia; Cardiac surgery; Iron; Infusion; Preoperative; Outcomes; Intravenous
| Introduction | ▴Top |
Anemia is common in cardiac surgical patients and is estimated to affect 25-40% of these patients [1-3]. The most common types of anemia seen in cardiac surgery are iron-deficiency anemia (up to 80% of all causes of anemia in cardiac surgery patients), anemia of chronic disease, and hospital-acquired anemia [2, 3]. Prior studies have shown that preoperative anemia is associated with increased blood transfusions, morbidity, mortality, and higher hospital costs [4-15]. Higher rates of stroke, acute renal injury, and total number of adverse postoperative outcomes have also been reported to be associated with preoperative anemia in cardiac surgery patients [13, 14].
Several studies have demonstrated the benefits of pharmacologic treatment of anemia with supplemental iron prior to non-cardiac surgery both in patients with iron-deficiency anemia and those without. In a prospective study, Diez-Lobo and colleagues showed that women with iron-deficiency anemia who received intravenous (IV) iron treatment 2 - 4 weeks before an abdominal hysterectomy had a 2.2 g/dL increase in preoperative hemoglobin (Hb) and 32% decrease in transfusion rates [16]. Froessler and colleagues performed a randomized controlled trial which showed that a single dose of IV iron in patients with iron-deficiency anemia before abdominal surgery reduced transfusion rates by 60% and shortened hospital length of stay by 2.7 days [17]. The intervention group had higher Hb levels prior to surgery and these levels increased to a greater degree 4 weeks after surgery. Cuenca and colleagues evaluated patients undergoing hip fracture surgery, regardless of whether they had iron-deficiency anemia preoperatively and found that preoperative IV iron administration was associated with a decrease in overall infection rates [18].
As mentioned, anemia is estimated to be present in 25-40% of cardiac surgery patients, and cardiac surgery itself is associated with a high rate of transfusion [1, 2, 19-22]. Loor et al had previously shown that cardiac surgical patients with anemia who require transfusion have higher morbidity and mortality [23]. Preoperative anemia in cardiac surgery patients may be due to several mechanisms. Ineffective red blood cell production secondary to chronic inflammation from the underlying pathology causes decreased production of and response to erythropoietin (EPO) in the bone marrow (anemia of chronic disease), inadequate storage of iron (absolute iron deficiency) or utilization of iron (functional iron deficiency) [24]. In a recent review on perioperative anemia management in cardiac surgery, Meybohm et al recommended that preoperative iron-deficiency anemia in cardiac surgery patients be corrected prior to surgery by the supplementation of iron in these patients [3]. They base this recommendation on the low risk profile of iron therapy and the high prevalence of anemia in cardiac surgery patients, although they do not do a full literature review of the current data as we offer in our systematic review.
However, the data on the use of IV iron supplementation for cardiac surgery patients are less robust than in other surgical subspecialties due to the limited number of randomized controlled trials and a lack of adequately powered studies.
Therefore, we performed a systematic review to assess and describe the current evidence of clinical efficacy and impact on patient outcomes of IV iron therapy as a prophylactic treatment of anemia in patients undergoing cardiac surgery. Our goal was to examine the quality of existing evidence on IV iron supplementation for preoperative anemia management. Understanding the current evidence could help clinicians design new clinical care pathways, IV iron clinics [25], and help understand the impact of anemia on patient and hospital outcomes, highlighting the interventions and resources needed to improve care of the cardiac surgical patient.
| Materials and Methods | ▴Top |
Protocol and registration
This systematic review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26]. A research librarian (David Osterbur, Countway Library of Medicine, Boston, MA) assisted in developing the protocol which was filed for registration through PROSPERO (submission identification number: 153900) at http://www.crd.york.ac.uk/PROSPERO.
A search of PubMed, Cochrane, CLINAHL, WOS, and EMBASE was performed on September 20, 2019 for all publications to date. The search term list (Supplementary Material 1, www.thejh.org) included the following terms: anemia, anemias, anemic, hemoglobin, or hematocrit; and preoperative, preoperative care, presurgical, presurgery, perisurgical, perisurgery, postoperative period, or postoperative care; iron, hematinics, antianemic agent, antianemic drug, antianemic factor, or antianemic therapy; and outcome assessment, outcome and process assessment, treatment outcome, patient outcome assessment, or outcome(s); not sickle cell anemia or sickle cell anemia or sickle.
The filters used to condense the results included human studies, English texts only, and type of publication such that case reports, abstracts, editorials, and comments were removed. Duplicate publications were also removed. Additional articles identified by other means were included such as clinical trial registrations identified with published results.
Inclusion/exclusion criteria
Included studies were limited to those that described patients undergoing cardiac surgery. Both prospective and retrospective studies were included. The literature review included studies in patients who were over 18 years of age and who received IV iron in the preoperative setting. Figure 1 shows the study flow diagram depicting inclusion and exclusion criteria and the overall literature selection process used.
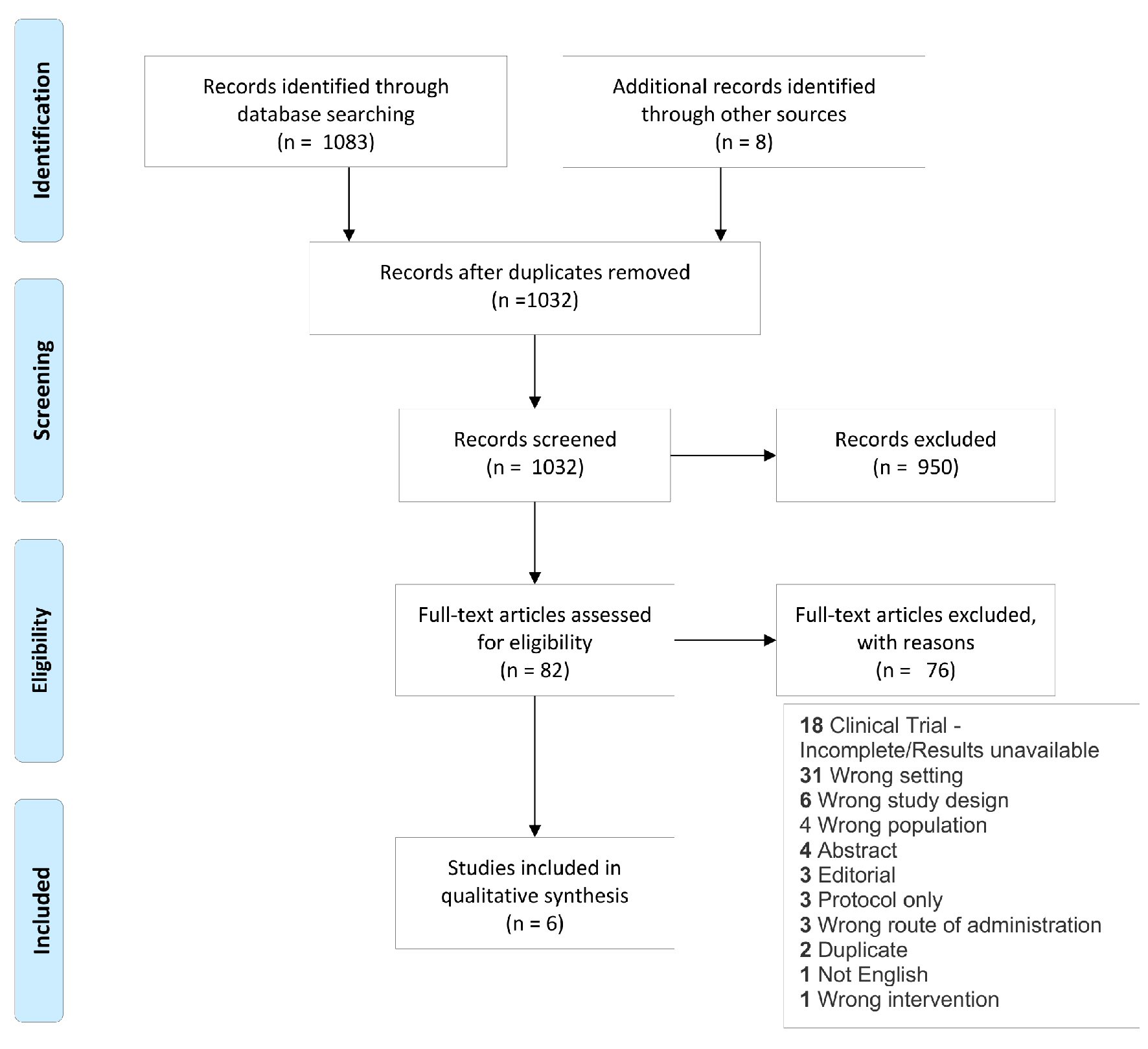 Click for large image | Figure 1. Study flow diagram showing literature selection criteria used for the systematic review. |
Study selection and data extraction
The publications included were selected via a systematic review. All citations using the above search terms in our selected databases were collated using Covidence software (Melbourne, Australia) [27]. All titles and abstracts were screened by one reviewer (BP) to determine eligible studies. Two reviewers (AB and RU) read the full text of each article that made it past the initial phase and independently decided if each publication should be included. Disagreements were resolved by consensus. After this, the first reviewer (BP) recorded the reasons for excluding each article which did not make it to this phase to determine validity of choices. When a final set of studies was chosen based on the above methods, one reviewer extracted data (BP) and another (KT) verified the data. The data we extracted included the name of the author(s), year of publication, design of the study, population characteristics, intervention(s), comparator(s), and outcome(s).
Outcomes
Our primary outcome of interest was change in Hb. The secondary outcomes of interest were transfusion rates, morbidity, safety, length of hospital stay, and mortality.
Data analysis
Once the included studies were chosen, we performed a qualitative analysis. We were unable to perform a meta-analysis due to the limited number of available studies. We present here a summary of the study characteristics, interventions, outcomes and associations, and limitations presented by these publications via our qualitative analysis.
Quality assessment
For the four randomized studies, quality was assessed using the Cochrane Risk of Bias Tool [28]. This scale is used to assess bias in randomized studies and includes rankings of “high,” “low,” or “unclear.” Studies are ranked on selection bias which is subcategorized as random sequence generation and allocation concealment; reporting bias which is referred to as selective reporting; other sources of bias; performance bias which is referred to as blinding of participants and personnel; detection bias which is referred to as blinding of outcome assessment; and attrition bias which is referred to as incomplete outcome data [28]. We defined study quality as “good” if the study had no “high” ratings for risk of bias. We defined the study quality as “fair” if the study had 1 - 2 “high” ratings for bias. We defined the study quality as “poor” if the study had 3 or more “high” ratings.
For the one non-randomized study, quality was assessed via the Newcastle-Ottawa scale. This scale is a tool to assess quality via a scoring system which rates studies based on selection, comparability, and outcome [29]. We defined study quality as “good” if the study scored in the ranges of 3 - 4 for selection, 1 - 2 for comparability, and 2 - 3 for outcome. We defined study quality as “fair” if the study scored 2 for selection, 1 - 2 for comparability, and 2 - 3 for outcome. We defined study quality as “poor” if the score did not meet criteria for either “good” or “fair.”
In an effort to decrease potential bias in study selection, we used the above criteria for inclusion and exclusion, performed multiple reviews, and reviewed reasons for exclusion of certain publications. Two reviewers independently assessed each study quality using the above scales and disagreements were resolved by consensus. We did not exclude studies based on how anemia was defined, sample size, or quality of the study. We used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria to score each publication based on the quantity and quality of studies and how consistent the findings were [30, 31].
| Results | ▴Top |
Literature search
A total of 1,083 articles were identified and reviewed. Of the 1,032 unique publications which were screened, 950 were excluded, and 82 full-text studies were assessed for eligibility. Of those, 76 were excluded, and six articles remained which met the criteria for this review (Fig. 1).
Study characteristics
Study characteristics for the six publications we identified can be found in Table 1 [32-37]. All included studies reported pre- and post-intervention Hb levels for iron therapy in the preoperative setting.
 Click to view | Table 1. Study Characteristics |
The studies were all conducted outside of the USA, with two being done in Spain, one in Canada, one in the UK, one in Denmark, and one in Switzerland [32-37]. The earliest publication year of the studies included was 2012. We included four single center, randomized, double-blinded prospective cohorts, one single center non-blinded non-randomized prospective cohort, and one single center non-blinded randomized prospective cohort. Across the six studies, there were 1,038 patients included. Four of the studies looked only at patients who had been diagnosed with preoperative anemia while the other two studies looked at only non-anemic patients at baseline. Four studies assessed patients undergoing either coronary artery bypass grafting (CABG) and/or valve replacement (VR) using cardiopulmonary bypass (CPB); one study looked only at VR on CPB, and one study looked only at interventional transcatheter VR without CPB. Given the limited number of studies, interventional approaches were not excluded. Three of the studies administered EPO along with iron as part of the intervention arm while three studies administered only iron. Each study administered iron intravenously at different times, ranging from 4 weeks preoperatively to 1 day preoperatively. The total doses of iron varied between studies, ranging from 1 - 5 total doses. Three studies used iron sucrose, one used iron isomaltose, and two used ferric carboxymaltose. Two studies compared the use of IV iron to oral iron therapy whereas the other four studies compared to placebo or historical control.
Definitions and measures
All of the studies defined anemia as Hb < 12 g/dL in women and < 13 g/dL in men in accordance with the World Health Organization (WHO) definition [38]. One study defined transfusion threshold as Hb < 7 g/dL, one defined it as < 7 g/dL or Hb 7 - 8 g/dL with symptomatic anemia, one defined it as < 7 - 8 g/dL intraoperatively and in the intensive care unit (ICU) or Hb < 8 g/dL on the inpatient ward, one defined it as < 8 g/dL if undergoing CABG or < 7 g/dL if undergoing only VR without CABG, and two studies did not define their transfusion thresholds. One study included intraoperative transfusion as part of their total transfusion requirement measurement, while the rest measured only the need for postoperative transfusion. Four studies included only patients who were anemic at baseline based on the above definition (regardless of the type of anemia) [32, 33, 35, 37]. Two of the studies only studied patients who were non-anemic at baseline by the above definition [34, 36].
Quality of included studies and risk for bias
The grading scheme by domain for each article is specified in Tables 2 and 3 [32-37]. Four studies were of good quality, one article was of fair quality, and one was of poor quality. The poor-quality publication was ranked as such due to high risk of bias for blinding and other sources of bias. The four articles of good quality had low risk of bias in blinding, randomization, and other sources of bias. The study which was of fair quality was the only non-randomized study we included in this review.
 Click to view | Table 2. Evidence Grading With Cochrane Risk of Bias Tool |
 Click to view | Table 3. Evidence Grading With Newcastle-Ottawa Scale Assessment of Study Quality |
Strength of evidence
The reported outcomes in this review were scored using the GRADE criteria. The evidence for Hb, transfusion rate, significant adverse events, infections, length of stay, and mortality were scored as Level 1b and of moderate quality. Reporting quality of life (QOL) as an outcome was scored as Level 2b and of low quality. The results are summarized in Table 4 [32-37].
 Click to view | Table 4. Strength of Evidence |
Association of iron therapy with Hb levels
Hb level before and after preoperative iron therapy were compared to assess the efficacy of this treatment (Table 5 [32-37]). Only one study reported significant difference in Hb between treatment and control groups after treatment. The study by Spahn et al [37], which included patients with anemia at baseline, reported a significant difference in Hb between groups on postoperative day 5 and day 7. Urena et al [34] did not find any significant difference in Hb levels between groups (intervention: 10.7 ± 1.2 g/dL to 10.9 ± 1.2 g/dL; control: 11.3 ± 1.6 g/dL to 11 ± 1.2 g/dL) from baseline; and this was the only study included in this review that looked only at transcatheter VR rather than VR via open surgery on cardiopulmonary bypass. Urena et al [34] only enrolled patients who were anemic at baseline and the intervention included EPO administration with iron treatment. Garrido-Martin et al [33] found no significant difference in Hb levels between the IV iron (14.0 ± 1.63 g/dL to 12.7 ± 1.63 g/dL) and placebo (14 ± 1.35 g/dL to 12.8 ± 1.29 g/dL) groups of patients without iron-deficiency anemia. This study also had a third comparator which was treatment with oral iron; this also showed no significant difference in Hb levels (13.7 ± 1.46 g/dL to 12.6 ± 1.70 g/dL) between either the IV iron or the control group. Johansson et al [36] did not report a significant change in Hb level in the intervention group (14.3 to 12.4 g/dL) 28 days after treatment compared to the placebo group (14.0 g/dL to 11.6 g/dL). Johansson et al excluded patients who were anemic at baseline and those who received transfusion intraoperatively.
 Click to view | Table 5. Hemoglobin Levels Before and After Preoperative Iron Therapy |
Cladellas et al [32] found significantly higher Hb levels in the treatment group (11 g/dL initial, 12.6 g/dL preoperatively) than the control (10.9 g/dL initial and preoperative Hb). Cladellas et al studied EPO given with iron as their treatment group. The placebo group and treatment group were also studied consecutively rather than concomitantly in time.
The study rated as poor by Padmanabhan et al [35] showed no significant change in Hb levels after treatment between groups (IV: 11.9 g/dl to 12.0 g/dl; oral: 11.4 g/dl to 11.8 g/dl). Only patients who were anemic at baseline were enrolled. The study was non-blinded, many of its patients were lost to follow-up, patients in the treatment arm could opt out of the second dose of IV iron, and the study changed its definition of anemia midway through the study to match WHO guidelines.
Association of iron therapy with transfusion rates
All six studies reported differences in transfusion rate as an outcome. These results are summarized in Table 6 [32-37]. Of the good quality studies, all four found no significant differences in transfusion rates between treatment and control groups. Urena et al [34], Garrido-Martin et al [33], and Johansson et al [36] did not find any significant difference in transfusion rates between groups. Padmanabhan et al [35] whose study we rate as poor quality did not find a significant difference in transfusion rates.
 Click to view | Table 6. Association of Iron Therapy With Transfusion Rate |
Cladellas et al [32] reported a significant difference in transfusion rates when comparing the treatment arm with the control group. They found a transfusion rate of 67% in the treatment group compared to 93% in the control group (P < 0.001). The nadir of Hb levels during cardiopulmonary bypass was significantly higher for patients in the treatment group and treatment was also associated with a lower morbidity and mortality. The number of patients requiring over six units of red blood cells also decreased significantly between groups from 36% in the control group to 4% in the treatment group, which indicates patients in the treatment group were less likely to require massive transfusion.
Spahn et al [37] also reported a significant difference in transfusion rates between the intervention and control group. The transfusion rate was 44% in the treatment group, with a median of 0 units (interquartile range (IQR): 0 to 2) transfused, compared to 53% transfusion rate in the control group (P < 0.05), with a median of 1 unit (IQR: 0 - 3) transfused. The transfusion rates for platelets and fresh frozen plasma were also measured during the same 7-day period, but not found to be significantly different between groups.
Association of iron therapy with other clinical outcomes
Results of secondary outcomes can be seen in Table 7 [32-37]. Four studies compared length of stay between groups. Urena et al [34] was the only publication of good quality which measured length of stay and found no difference between groups. Cladellas et al [32] which we rate as fair quality studied length of stay and found there to be a significantly shorter admission (P < 0.01; 10 days, IQR: 8 - 14; 15 days, IQR: 10 - 27) in patients who received IV iron. Padmanabhan et al [35] whose study we rank as poor quality found no difference in length of stay between groups.
 Click to view | Table 7. Other Outcomes |
Five studies assessed mortality between groups. Cladellas et al [32] was of fair quality and did find a significant difference in mortality with a 9% mortality rate in the treatment group compared to a 23% mortality rate in the control group (P = 0.04). The five other studies in this review reported no mortality difference between groups.
All studies assessed infection rates between treatment and control groups. Cladellas et al [32] which was of fair quality was the only study which showed a significant difference between groups. They reported an 8% incidence of infections in the treatment group versus 24% in the control group (P = 0.01). The five other studies did not find any significant difference of infection rates between groups.
One study assessed QOL between groups. Padmanabhan et al [35] did not find a significant difference in QOL between groups as measured by the modified Short Form-36 and EUROQOL-5D questionnaires.
All studies recorded adverse events. None of the studies reported significant differences between groups regarding adverse events as defined by each individual study.
| Discussion | ▴Top |
Anemia in cardiac surgery has previously been shown to be a risk factor for increased need for transfusions, adverse outcomes, mortality, and higher costs to the patient and hospital system [1, 2, 4, 8, 11]. The use of IV iron therapy preoperatively has been studied in other surgical subspecialties with promising results; however its use in cardiac surgery has been poorly defined [16-18]. Shin et al who published a systematic review in 2019, showed that the current evidence for iron therapy preoperatively in orthopedic surgery patients supports its use to decrease number of transfusions, length of stay, and infections although without changing mortality [39]. The goal of our paper was to review the current evidence regarding the use of preoperative iron therapy in cardiac surgery. Our review is the most recent and comprehensive on this topic, and we conclude that more work needs to be done to elucidate whether there is significant benefit from treating cardiac surgery patients with preoperative iron. Hogan et al published a systematic literature review in 2014 looking at the effects of anemia on outcomes in cardiac surgery [40]. Although they reported some of the same studies, their search included preoperative and postoperative iron administration, including co-administration of EPO. Our review is focused on iron therapy as a preoperative intervention and its effects on outcomes after cardiac surgery.
Three of the six studies (Johansson et al [36], Cladellas et al [32], and Spahn et al [37]) found significantly higher Hb levels in the treatment group than the control group. However, the studies included were of mixed quality and at most of moderate quality evidence. There currently remains a lack of strong evidence for IV iron therapy in optimizing Hb levels prior to cardiac surgery.
Cladellas et al [32] and Spahn et al [37] found a significant difference in transfusion rates in the treatment group. However, Cladellas et al [32] was the only study to find significant differences in secondary outcomes. Anemic patients were found to have a greater than three-fold increase in the odds of death and four-fold increase in odds of major complications. There was a significant decrease in acute renal failure, congestive heart failure, prolonged intubation, and composite morbidity in the treatment group.
There are several possible explanations for the differences across studies. The dosage, duration, and formulation of iron therapy differed across studies. The studies reporting no significant difference in outcomes with iron therapy address this discrepancy by discussing the possibility of not having administered IV iron in high enough dose to produce a significant effect. In fact, participants in the study by Padmanabhan et al [35] could opt out of the second dose of iron and therefore only receive one dose of IV iron. The study of Cladellas et al [32], which was the only publication to find significant differences in multiple outcomes, used fie doses of IV iron over a 4-week preoperative treatment period and focused on valve repair only. This was the highest number of IV iron doses over the longest period preoperatively of any of the studies. Thus, it is possible that the significance of their findings with iron therapy may have been related to the number of iron doses over a longer period of time preoperatively compared to the other studies. It is possible that other studies may not have given a high enough dose to identify significant differences between groups, or that the broad scope of cardiac surgery introduces more heterogeneity.
Side effects of IV iron, including infection, hypersensitivity, and iron overload, have been reported [3]. One systematic review of IV iron reported infusion reactions at the IV site [41]. However, all six studies in this review reported no adverse events and no significant increase in infection rates between groups. Cladellas et al [32] found a significantly lower rate of infection in the IV iron group compared to historical control. These findings support the current literature citing no significant association between IV iron therapy and increased infection rates [41-45].
There were three types of IV iron formularies used in the reported studies. Three studies involved administration of iron sucrose [32-34]. Johansson et al [36] administered iron isomaltose, while Padmanabhan et al [35] and Spahn et al [37] administered ferric carboxymaltose. Ferric carboxymaltose is reported to have effects from a single dose and has higher maximum concentration per dosage [46]. Few studies compare efficacy of formulation, and none in the cardiac surgery literature. In regard to hypersensitivity reactions, current formulations of IV iron including iron sucrose, iron isomaltose, and ferric carboxymaltose have been shown to be much better tolerated than prior formulations of iron therapy such as iron dextran [47].
Two of the studies assessed the route of iron administration. Garrido-Martin et al [33] and Padmanabhan et al [35] compared iron therapy via the IV route with the oral route, although they did not find any difference between either route or placebo. The use of oral iron may not be as appropriate for use in cardiac surgery, as oftentimes cardiac surgery is urgent and there may not be enough time to reach a therapeutic threshold with oral iron. The IV route has a quicker onset and avoids first-pass metabolism. Oral iron may be less favorable due to gastrointestinal intolerance, and reduced uptake during inflammatory states [48].
The role of EPO and whether it may have impacted results is unclear. It should be noted that three of these studies looked at the concomitant administration of EPO with iron (Cladellas et al [32], Urena et al [34], and Spahn et al [37]), while the three other studies looked at iron therapy alone. Yoo et al recently conducted a study looking primarily at the effect of EPO on patients undergoing cardiac surgery and showed that treatment with a single dose of EPO with a small dose of iron 1 day before surgery reduced transfusion rates [49]. A recent meta-analysis by Kei and colleagues reported decreased transfusions rates when comparing IV iron with and without EPO, although the sub-group analysis included cardiac and orthopedic surgeries [45, 50]. Theoretic risks of EPO include an increase in thrombosis, although there is a lack of clear corroborating evidence. Furthermore, a recent study showed that patients undergoing cardiac surgery receiving EPO showed no differences in thrombosis, infection, or mortality [51].
A notable difference among studies is the state of preoperative anemia. Most of the studies investigated patients who were anemic at baseline (regardless of type of anemia); however Johansson et al [36] and Garrido-Martin et al [33] studied only non-anemic patients. Many cardiac surgery patients are anemic at baseline, and it is well established that the Hb level affects transfusion rates and outcomes after cardiac surgery [1-3]. Furthermore, blood management protocols recommend iron therapy for treatment of iron-deficiency anemia [52]. The lack of consistent anemia delineation likely diminishes the study results. Of the four studies which looked only at patients who were anemic at baseline (regardless of type of anemia), the degree of preoperative anemia as measured by baseline Hb was similar across the studies. Thus, we are unable to comment on the efficacy of IV iron based on severity of preoperative anemia in those studies which looked only at anemic patients. Given that the studies looked at patients with all types of anemia, we are unable to comment on the efficacy of treatment with IV iron based on the type of preoperative anemia.
Other methods of reducing rates of transfusion aside from iron therapy include cell salvage intraoperatively, autologous blood transfusions, restrictive institutional blood transfusion guidelines, and decreasing the number of labs drawn in the ICU and postoperatively. A recent review article by Meybohm et al on the management of perioperative anemia in cardiac surgery suggests that “correcting preoperative anemia should be mandatory ahead of planned cardiac surgery” and “preoperative therapy of anemia with parenteral iron is recommended according to current evidence” [3]. The authors based this recommendation on the low risk profile of iron therapy and the high prevalence of anemia in cardiac surgery patients, although they do not do a full literature review of the current data as we offer in our systematic review.
Our review shows that the current state of the evidence on iron therapy prior to cardiac surgery has not yet been clearly defined and more evidence is required to strengthen consensus statements and blood management systems. The six studies we identified via our systematic review are of mixed quality and mixed results with half of the studies showing a significant difference in Hb levels and only one study showing significant differences in transfusion or any other outcome. More work needs to be done to make any definitive conclusions, which can guide consensus guidelines on treatment of cardiac surgery patients preoperatively with IV iron.
Strengths and limitations
Our systematic review has several strengths. We performed a comprehensive search with broad search terms, enlisted methodology expertise, and did not limit the search by timeframe. We included experimental designs to avoid selection bias. We assessed quality by Cochrane Risk of Bias Tool and the Newcastle-Ottawa Scale Assessment of Study Quality. Our review is currently the most recent and comprehensive on this topic.
One of the significant limitations of our review is the paucity of publications investigating iron therapy as an intervention preoperatively prior to cardiac surgery. We were only able to identify six studies which met our inclusion and exclusion criteria. Of these studies, only four of the six studies were of good quality based on our quality assessment and risk of bias scores, one was of fair quality, and one was poor quality. Results were reported on all studies despite the quality. Nevertheless, strong conclusions could not be drawn from these six studies. Individual studies may not have given high enough doses of the treatment or have been adequately powered to identify significant differences between groups.
The study done by Urena et al [34] was only for percutaneous VR without cardiopulmonary bypass while the other studies looked only at open cardiac surgery on bypass. The blood loss and coagulopathy with cardiopulmonary bypass is much higher than without and thus the study by Urena et al may not be comparable to the other four studies.
The studies included in this paper also differ in whether they included or excluded patients with preoperative anemia which limits the ability to generalize results to all patients regardless of baseline Hb levels.
None of these studies were done in the USA, which may make the results less generalizable to the US hospital system given changes in practice across countries. We excluded studies looking at the pediatric population and those related to sickle cell anemia. There are also ongoing relevant studies, although no published data are yet available [53].
| Conclusions | ▴Top |
More studies are needed to determine the effect of preoperative iron therapy in cardiac surgery. This is especially important because cardiac surgical patients receive more blood transfusions than those undergoing other types of surgery; and blood transfusion in these patients has been associated with higher morbidity and mortality [54]. Only half of the studies in our review showed higher Hb levels with the use of iron therapy, and only one of the six studies showed significant differences in multiple other outcomes such as transfusion rate and morbidity. The study which showed significant differences in multiple outcomes with iron therapy (Cladellas et al [32]) gave participants the highest dose of iron over the longest period preoperatively, and studied patients undergoing valve repairs. It is possible that the other five studies did not give enough iron over a long enough treatment time to see more significant differences in outcomes, or the data are confounded by the use of bypass in nonvalvular surgeries. More studies are needed to investigate the role of iron in cardiac surgery. Other interventions such as the use of EPO, vitamin B12, and folate may also play a role and offer areas for future investigation as well. The investigation of ways to reduce transfusion rates in cardiac surgery is important in order to decrease costs to both the patient and the hospital. Based on our review of the current literature, we are unable to recommend that patients undergoing cardiac surgery be routinely treated preoperatively with IV iron.
| Supplementary Material | ▴Top |
Suppl 1. Search terms used in each database.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
Dr. Urman reports unrelated funding from Merck, Medtronic, Takeda, Heron, AcelRx, NSF, and AHRQ. Other authors report no conflicts of interest.
Author Contributions
Each author certifies involvement in data acquisition, manuscript preparation and editing.
Data Availability
Literature search data are available upon request.
| References | ▴Top |
- Kulier A, Levin J, Moser R, Rumpold-Seitlinger G, Tudor IC, Snyder-Ramos SA, Moehnle P, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116(5):471-479.
doi pubmed - Karkouti K, Wijeysundera DN, Beattie WS, Reducing Bleeding in Cardiac Surgery I. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation. 2008;117(4):478-484.
doi pubmed - Meybohm P, Westphal S, Ravn HB, Ranucci M, Agarwal S, Choorapoikayil S, Spahn DR, et al. Perioperative Anemia Management as Part of PBM in Cardiac Surgery - A Narrative Updated Review. J Cardiothorac Vasc Anesth. 2020;34(4):1060-1073.
doi pubmed - Theusinger OM, Kind SL, Seifert B, Borgeat L, Gerber C, Spahn DR. Patient blood management in orthopaedic surgery: a four-year follow-up of transfusion requirements and blood loss from 2008 to 2011 at the Balgrist University Hospital in Zurich, Switzerland. Blood Transfus. 2014;12(2):195-203.
- Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion. 2014;54(10 Pt 2):2617-2624.
doi pubmed - Roubinian NH, Escobar GJ, Liu V, Swain BE, Gardner MN, Kipnis P, Triulzi DJ, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion. 2014;54(10 Pt 2):2678-2686.
doi pubmed - Leahy MF, Roberts H, Mukhtar SA, Farmer S, Tovey J, Jewlachow V, Dixon T, et al. A pragmatic approach to embedding patient blood management in a tertiary hospital. Transfusion. 2014;54(4):1133-1145.
doi pubmed - Goodnough LT, Maggio P, Hadhazy E, Shieh L, Hernandez-Boussard T, Khari P, Shah N. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion. 2014;54(10 Pt 2):2753-2759.
doi pubmed - Gross I, Seifert B, Hofmann A, Spahn DR. Patient blood management in cardiac surgery results in fewer transfusions and better outcome. Transfusion. 2015;55(5):1075-1081.
doi pubmed - Moskowitz DM, McCullough JN, Shander A, Klein JJ, Bodian CA, Goldweit RS, Ergin MA. The impact of blood conservation on outcomes in cardiac surgery: is it safe and effective? Ann Thorac Surg. 2010;90(2):451-458.
doi pubmed - Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, Hamdorf J, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57(6):1347-1358.
doi pubmed - Meybohm P, Muellenbach RM, Keller H, et al. Patient blood management in der Herzchirurgie. Z Herz-Thorax-Gefαβchir. 2017;31:247-265.
doi - Froessler B, Rueger AM, Connolly MP. Assessing the costs and benefits of perioperative iron deficiency anemia management with ferric carboxymaltose in Germany. Risk Manag Healthc Policy. 2018;11:77-82.
doi pubmed - Trentino KM, Farmer SL, Swain SG, Burrows SA, Hofmann A, Ienco R, Pavey W, et al. Increased hospital costs associated with red blood cell transfusion. Transfusion. 2015;55(5):1082-1089.
doi pubmed - Goodnough LT, Shieh L, Hadhazy E, Cheng N, Khari P, Maggio P. Improved blood utilization using real-time clinical decision support. Transfusion. 2014;54(5):1358-1365.
doi pubmed - Diez-Lobo AI, Fisac-Martin MP, Bermejo-Aycar I, et al. Preoperative intravenous iron administration corrects anemia and reduces transfusion requirement in women undergoing abdominal hysterectomy. Transfus Alternat Transfus Med. 2007;9:114-119.
doi - Froessler B, Palm P, Weber I, Hodyl NA, Singh R, Murphy EM. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Ann Surg. 2016;264(1):41-46.
doi pubmed - Cuenca J, Garcia-Erce JA, Martinez F, Perez-Serrano L, Herrera A, Munoz M. Perioperative intravenous iron, with or without erythropoietin, plus restrictive transfusion protocol reduce the need for allogeneic blood after knee replacement surgery. Transfusion. 2006;46(7):1112-1119.
doi pubmed - Tinegate H, Pendry K, Murphy M, Babra P, Grant-Casey J, Hopkinson C, Hyare J, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion. 2016;56(1):139-145.
doi pubmed - Arias-Morales CE, Stoicea N, Gonzalez-Zacarias AA, Slawski D, Bhandary SP, Saranteas T, Kaminiotis E, et al. Revisiting blood transfusion and predictors of outcome in cardiac surgery patients: a concise perspective. F1000Res. 2017;6:168.
doi pubmed - Robich MP, Koch CG, Johnston DR, Schiltz N, Chandran Pillai A, Hussain ST, Soltesz EG. Trends in blood utilization in United States cardiac surgical patients. Transfusion. 2015;55(4):805-814.
doi pubmed - Geissler RG, Rotering H, Buddendick H, Franz D, Bunzemeier H, Roeder N, Kwiecien R, et al. Utilisation of blood components in cardiac surgery: a single-centre retrospective analysis with regard to diagnosis-related procedures. Transfus Med Hemother. 2015;42(2):75-82.
doi pubmed - Loor G, Rajeswaran J, Li L, Sabik JF, 3rd, Blackstone EH, McCrae KR, Koch CG. The least of 3 evils: exposure to red blood cell transfusion, anemia, or both? J Thorac Cardiovasc Surg. 2013;146(6):1480-1487 e1486.
doi pubmed - Abraham J, Sinha R, Robinson K, Scotland V, Cardone D. Aetiology of preoperative anaemia in patients undergoing elective cardiac surgery-the challenge of pillar one of Patient Blood Management. Anaesth Intensive Care. 2017;45(1):46-51.
doi pubmed - Klein AA, Chau M, Yeates JA, Collier T, Evans C, Agarwal S, Richards T, et al. Preoperative intravenous iron before cardiac surgery: a prospective multicentre feasibility study. Br J Anaesth. 2020;124(3):243-250.
doi pubmed - Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
doi pubmed - Covidence. Systematic review management software. Melbourne, Australia. Available at: https://www.covidence.org. Accessed: January 8, 2020.
- Higgins JPT, Green S, Cochrane Collaboration, editors. Cochrane handbook for systematic reviews of interventions. Chichester, England. Higgins JPT, Green S: The cochrane handbook for systematic reviews of interventions 4.2.5. Available at: http://www.cochrane.org/resources/handbook/hbook.htm. Accessed January 9, 2020.
- Wells G, Shea B, O'Connell J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 9, 2020.
- Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, Phillips B, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64(5):669-677.
doi pubmed - Eden J, Levit L, Berg A, et al (editors). Finding what works in health care: standards for systematic reviews. Washington, DC: National Academies Press (US). 2011. Available at: https://www.ncbi.nlm.nih.gov/books/NBK209518/. Accessed January 9, 2020.10.17226/13059
- Cladellas M, Farre N, Comin-Colet J, Gomez M, Merono O, Bosch MA, Vila J, et al. Effects of preoperative intravenous erythropoietin plus iron on outcome in anemic patients after cardiac valve replacement. Am J Cardiol. 2012;110(7):1021-1026.
doi pubmed - Garrido-Martin P, Nassar-Mansur MI, de la Llana-Ducros R, Virgos-Aller TM, Rodriguez Fortunez PM, Avalos-Pinto R, Jimenez-Sosa A, et al. The effect of intravenous and oral iron administration on perioperative anaemia and transfusion requirements in patients undergoing elective cardiac surgery: a randomized clinical trial. Interact Cardiovasc Thorac Surg. 2012;15(6):1013-1018.
doi pubmed - Urena M, Del Trigo M, Altisent OA, Campelo-Prada F, Regueiro A, DeLarochelliere R, Doyle D, et al. Combined erythropoietin and iron therapy for anaemic patients undergoing transcatheter aortic valve implantation: the EPICURE randomised clinical trial. EuroIntervention. 2017;13(1):44-52.
doi pubmed - Padmanabhan H, Siau K, Nevill AM, Morgan I, Cotton J, Ng A, Brookes MJ, et al. Intravenous iron does not effectively correct preoperative anaemia in cardiac surgery: a pilot randomized controlled trial. Interact Cardiovasc Thorac Surg. 2019;28(3):447-454.
doi pubmed - Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer(R)) reduces postoperative anaemia in preoperatively non-anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double-blind placebo-controlled clinical trial (the PROTECT trial). Vox Sang. 2015;109(3):257-266.
doi pubmed - Spahn DR, Schoenrath F, Spahn GH, Seifert B, Stein P, Theusinger OM, Kaserer A, et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet. 2019;393(10187):2201-2212.
doi - World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2001. Available at: https://apps.who.int/iris/handle/10665/85839. Accessed January 9, 2020.
- Shin HW, Park JJ, Kim HJ, You HS, Choi SU, Lee MJ. Efficacy of perioperative intravenous iron therapy for transfusion in orthopedic surgery: A systematic review and meta-analysis. PLoS One. 2019;14(5):e0215427.
doi pubmed - Hogan M, Klein AA, Richards T. The impact of anaemia and intravenous iron replacement therapy on outcomes in cardiac surgery. Eur J Cardiothorac Surg. 2015;47(2):218-226.
doi pubmed - Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter-Gvili A. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clin Proc. 2015;90(1):12-23.
doi pubmed - Brewster UC, Coca SG, Reilly RF, Perazella MA. Effect of intravenous iron on haemodialysis catheter microbial colonization and blood-borne infection. Nephrology (Carlton). 2005;10(2):124-128.
doi pubmed - Aronoff GR, Bennett WM, Blumenthal S, Charytan C, Pennell JP, Reed J, Rothstein M, et al. Iron sucrose in hemodialysis patients: safety of replacement and maintenance regimens. Kidney Int. 2004;66(3):1193-1198.
doi pubmed - Pieracci FM, Henderson P, Rodney JR, Holena DN, Genisca A, Ip I, Benkert S, et al. Randomized, double-blind, placebo-controlled trial of effects of enteral iron supplementation on anemia and risk of infection during surgical critical illness. Surg Infect (Larchmt). 2009;10(1):9-19.
doi pubmed - Torres S, Kuo YH, Morris K, Neibart R, Holtz JB, Davis JM. Intravenous iron following cardiac surgery does not increase the infection rate. Surg Infect (Larchmt). 2006;7(4):361-366.
doi pubmed - Onken JE, Bregman DB, Harrington RA, Morris D, Acs P, Akright B, Barish C, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54(2):306-315.
doi pubmed - Auerbach M, Macdougall I. The available intravenous iron formulations: History, efficacy, and toxicology. Hemodial Int. 2017;21(Suppl 1):S83-S92.
doi pubmed - Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ. 2013;347:f4822.
doi pubmed - Yoo YC, Shim JK, Kim JC, Jo YY, Lee JH, Kwak YL. Effect of single recombinant human erythropoietin injection on transfusion requirements in preoperatively anemic patients undergoing valvular heart surgery. Anesthesiology. 2011;115(5):929-937.
doi pubmed - Kei T, Mistry N, Curley G, Pavenski K, Shehata N, Tanzini RM, Gauthier MF, et al. Efficacy and safety of erythropoietin and iron therapy to reduce red blood cell transfusion in surgical patients: a systematic review and meta-analysis. Can J Anaesth. 2019;66(6):716-731.
doi pubmed - Duce L, Cooter ML, McCartney SL, Lombard FW, Guinn NR. Outcomes in patients undergoing cardiac surgery who decline transfusion and received erythropoietin compared to patients who did not: a matched cohort study. Anesth Analg. 2018;127(2):490-495.
doi pubmed - Goodnough LT, Shander A. Patient blood management. Anesthesiology. 2012;116(6):1367-1376.
doi pubmed - ClinicalTrials.gov. Intravenous Iron for Treatment of Anaemia Before Cardiac Surgery (ITACS) NCT02632760. Available at: https://clinicaltrials.gov/ct2/show/NCT02632760. Accessed March 20, 2020.
- D'Souza S, Satarkar B, Bharne SS. Anaesthesia for a minor procedure in a patient with fontan physiology. Indian J Anaesth. 2012;56(6):572-574.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


