| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 9, Number 4, December 2020, pages 123-131
Treatment-Related Mortality From Infectious Complications in an Acute Leukemia Clinic
Jorge Torres-Floresa, Ramiro Espinoza-Zamoraa, Jorge Garcia-Mendezb, Eduardo Cervera-Ceballosc, Alejandro Sosa-Espinozac, Nidia Zapata-Cantoa, d
aHematology Department, Instituto Nacional de Cancerologia Mexico (INCan), Mexico City, Mexico
bInfectious Diseases Department, Instituto Nacional de Cancerologia Mexico (INCan), Mexico City, Mexico
cTeaching Department, Instituto Nacional de Cancerologia Mexico (INCan), Mexico City, Mexico
dCorresponding Author: Nidia Zapata-Canto, Instituto Nacional de Cancerologia Mexico (INCan), San Fernando Avenue 22, Col. Seccion XVI Tlalpan, 14080 Mexico City, Mexico
Manuscript submitted September 15, 2020, accepted October 29, 2020, published online November 6, 2020
Short title: Infections in Acute Leukemia Patients
doi: https://doi.org/10.14740/jh751
| Abstract | ▴Top |
Background: The main causes of mortality in patients with acute leukemia are the infectious complications. The author wanted to know the induction-related mortality and treatment-related mortality in the acute leukemia patients at the Instituto Nacional de Cancerologia (INCan), Mexico. Also the author is interested in finding out the micro-organism and the main site of infection to make some changes in the management of patients in these clinics. Primary objective was induction chemotherapy-related mortality and treatment-related mortality. Secondary objective was to determine the site of infection, micro-organism, type of chemotherapy related with more mortality and relapse mortality.
Methods: This was a retrospective case-series analysis of all patients who were admitted to the INCan Acute Leukemia Clinic between January 2012 and December 2015 with febrile neutropenic complications. We reviewed the case histories of all patients, including those with acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia (AML), acute biphenotypic leukemia and acute promyelocytic leukemia, regardless of disease status (newly diagnosed or relapsed) at the time of clinic attendance. Patients who died as the result of an infectious complication during the analysis window were identified, and their demographics, disease characteristics, treatment history (chemotherapy within 45 days of date of death) and details of the infectious complication resulting in death were collected.
Results: Of the 313 patients studied during that time period, 84 (27%) died as a result of infectious complications. Lung infections were the most common, accounting for 67% of all deaths from infectious complications. Escherichia coli producing extended-spectrum beta-lactamases was the most frequently isolated infectious organism (12 patients; 14%). The majority of deaths occurred during either induction therapy (27 patients; 32%) or treatment for a first relapse (25 patients; 30%). Hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone (hyper-CVAD) was the chemotherapy regimen most commonly received within 45 days prior to death (17 patients; 20%).
Conclusions: Our findings suggest a need for long-term management and supportive care to prevent infectious complication-associated fatalities during both initial chemotherapy and subsequent disease relapse in patients with acute leukemia. The use of prophylaxis will help patients to prevent complications.
Keywords: Bacteremia; Chemotherapy; Febrile neutropenia; Infections; Leukemia; Neutropenia; Prophylaxis
| Introduction | ▴Top |
Infectious complications are a major cause of morbidity [1] and mortality [2] in patients with hematologic malignancies, including acute leukemia. Patients with hematologic malignancies are at increased risk of infections owing to both the effects of the disease on the immune system and the immunotoxic effects of chemotherapy, most notably neutropenia and febrile neutropenia [2-4]. Many common regimens administered for the treatment of acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia (AML) and other acute leukemias are highly myelosuppressive, and thereby result in high rates of severe neutropenia. Patients receiving treatment with these regimens are thus also at increased risk of febrile neutropenia, a medical emergency requiring immediate attention [2].
The management of febrile neutropenic patients is determined by a risk-stratification approach [5]. The standard treatment strategy for severe neutropenia or febrile neutropenia in patients with hematologic malignancies is to administer broad-spectrum antibiotics immediately following diagnostic blood culture and other culture collection [2, 6]. Recommendations in the United States National Comprehensive Cancer Network guidelines on Prevention and Treatment of Cancer-Related Infections [7, 8] include the selection of initial antibiotic therapy based on factors including broad-spectrum coverage, the potential infecting organisms (including multi-drug-resistant organisms) and the site of infection, followed by targeted treatment of documented infections. The guidelines also provide recommendations for antibacterial, antifungal and antiviral prophylaxis, particularly for patients at high risk of infection [8].
Despite the best practices outlined above, infectious complications remain a prominent cause of mortality in patients with acute leukemia. In the context of the evolving range of infectious micro-organisms that may result in disease, including the emergence and spread of various multi-drug-resistant organisms, we conducted a retrospective analysis of patients with infectious complications seen in the Acute Leukemia Clinic at the Instituto Nacional de Cancerologia (INCan), in Mexico City. Our analysis was intended to determine common organisms associated with treatment-related mortality resulting from infectious complications, the common sites of infection and the chemotherapy regimens most frequently associated with such complications.
| Materials and Methods | ▴Top |
This was a retrospective case-series analysis of all patients who were admitted to the INCan Acute Leukemia Clinic between January 2012 and December 2015 with febrile neutropenic complications. We reviewed the case histories of all patients, including those with ALL, AML, acute biphenotypic leukemia and acute promyelocytic leukemia, regardless of disease status (newly diagnosed or relapsed) at the time of clinic attendance. Patients who died as the result of an infectious complication during the analysis window were identified, and their demographics, disease characteristics, treatment history (chemotherapy within 45 days of date of death) and details of the infectious complication resulting in death were collected. As patients with ALL or AML comprised the vast majority of the study population, additional analyses were performed for patients in these subgroups. All patients had Eastern Cooperative Oncology Group(ECOG) 0-2, were able to received intensive chemotherapy, written informed consent was obtained to all patients in accordance with the Declaration of Helsinki previous to receive chemotherapy. Karyotype was used to classify cytogenetic risk. The investigational committee accept this retrospective study.
Statistical analyses were performed using SAS® 9.3 software (SAS Institute Incorporated, North Carolina, USA). Continuous variables were summarized using means and standard deviations, and compared between the two leukemia subtypes using Student’s t-test; categorical variables were summarized using numbers and percentages, and compared using a Chi-squared test. Survival outcomes from diagnosis were analyzed using Kaplan-Meier methodology, and compared between leukemia types using the log-rank test. Hazard ratios (HRs), denoting the risk of death or occurrence of leukemia, were estimated with their 95% confidence intervals (CIs) using a Cox proportional hazards model. For all statistical tests, two-sided P values < 0.05 were considered significant.
Infectious organisms were identified from blood obtained by peripheral venipuncture and central venous catheter, urine, feces and/or bronchoscopy culture within the 5 days prior to death. Blood cultures were performed in patients with fever (temperature > 38 °C), and were repeated every 3 days in cases of persistent fever. Urine examination with culture was performed in all patients with febrile neutropenia, and feces culture in all patients with diarrhea. Expectoration culture was performed in patients with cough and secretions, with bronchoscopy being carried out in the event of major pulmonary infection. Microorganisms were identified by means of matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (BD™ Bruker MALDI Biotyper System; Bruker Daltonics, Billerica, MA, USA). Antimicrobial susceptibility testing was carried out by the Clinical and Laboratory Standards Institute using the BD Phoenix™ Microbiology System (Phoenix, Bruker Daltonics, Sparks, MD, USA).
| Results | ▴Top |
Patients
A total of 313 Mexican mestizos patients were admitted to the INCan Acute Leukemia Clinic with febrile neutropenic complications between January 2012 and December 2015. Of these 313 patients, 187 presented with ALL, 100 with AML, 12 with promyelocytic leukemia, 10 with biphenotypic leukemia, two with acute leukemia secondary to treatment and one each with blast-phase chronic granulocytic leukemia and a blastic plasmacytoid neoplasm. All 313 patients had received chemotherapy as part of their treatment for acute leukemia. Ninety-six patients died during the study window; 84 of these deaths were attributable to infectious complications. Thus, the mortality rate owing to treatment-related infectious complications in our series was 26.8%. Of the 12 patients whose deaths were not attributable to infectious complications, four died at home while receiving palliative care, five died as a result of disseminated intravascular coagulation and one each died from tumor lysis syndrome, hepatic insufficiency and cardiogenic shock.
The characteristics of the 84 patients who died from infectious complications are summarized in Table 1 [9-11]. Fifty-two (61.9%) of the patients were male, with similar numbers having relapsed (43 patients; 51.2%) versus treatment-naive leukemia (41 patients; 48.8%). The most common leukemia types were ALL (51 patients; 60.7%) and AML (22 patients; 26.2%), and the median age of the population was 35.2 years (range 15.8 - 71.3 years). Of the 84 patients, 13 patients (15%) had no karyotype, two patients (2.2%) had low cytogenetic risk, 11 patients had intermediate risk (13.3%) and 58 patients had his cytogenetic risk (69.5%). Leukocyte count ranged 19,000/mm3 (100 - 378,000/mm3) and neutrophil counts ranged 3,500/mm3 (0 - 90,000/mm3). More than half of all patients who died as a result of infectious complications were receiving either induction therapy (27 patients; 32.1%) or treatment for first relapse (25 patients; 29.8%) at the time of death. Nine patients (10.7%) were at the consolidation stage, while only two (2.4%) were receiving maintenance therapy and one (1.2%) was receiving palliative care. Nine (10.7%) patients had refractory leukemia that had failed to respond to prior regimens (median two prior regimens).
 Click to view | Table 1. Characteristics of Patients Who Died of Infectious Complications Between January 2012 and December 2015 |
Infectious complications
Of the 84 patients who died as a result of infectious complications, the cause of death was identified as septic shock in 75 (89.3%) patients and acute respiratory insufficiency in the remaining nine (10.7%). As shown in Table 2, the lung was the most common primary infection site (56 patients; 66.7%), followed by the skin (nine patients; 10.7%) and the gastrointestinal tract (eight patients; 9.5%).
 Click to view | Table 2. Site/Nature of Infection in Patients Who Died of Infectious Complications |
Table 3 lists the infectious organisms isolated in the 84 patients who died of infectious complications. The most common organisms were: Escherichia coli producing extended-spectrum beta-lactamases (ESBL) (12 patients; 14.3%); Enterococcus faecium (seven patients; 8.3%); Pseudomonas aeruginosa (non-multi-drug resistant) (five patients; 6.0%). The most commonly identified fungal infections were Candida albicans (four patients; 4.8%) and Candida glabrata (two patients; 2.4%). In 29 patients (34.5%), the culture failed to develop and no organism was identified.
 Click to view | Table 3. Most Frequently Isolated Organisms From Blood, Urine, Feces, or Bronchoscopy Culture in Patients Who Died of an Infectious Complication, Depending of the Different Type of Chemotherapy and the Number of Treatment in Correlation With the Site and the Microorganism of Infection |
Chemotherapy regimens
The chemotherapy regimen most commonly administered to the 84 patients within 45 days of their death from infectious complications was hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone (hyper-CVAD; received by 17 patients (20.2%)). The cytarabine/daunorubicin 7+3 regimen and palliative 6-mercaptopurine, vincristine, methotrexate and prednisone (POMP) were each received by 10 patients (11.9%). Twenty-one (25.0%) patients received no chemotherapy within 28 days of their death, being either in receipt of antibiotic therapy or experiencing disease relapse during this period. Details of the chemotherapy regimens administered within 45 days of the death of those patients who died of infectious complications are summarized in Table 1.
Management and outcomes of infectious complications
In 59 (70.2%) of the 84 patients who died of infectious complications, death occurred during an episode of febrile neutropenia in a median of 11 days (range 0 - 64 days) from the last dose of chemotherapy. All patients received antibiotics and granulocyte colony-stimulating factor. Forty-eight patients (58.3%) presented at the intensive care unit (ICU) with septic shock, but only 24 (28.5%) patients actually received ICU treatment. Thirty-three (26.1%) patients declined ICU treatment for economic reasons, while 11 (13%) were transferred to palliative care. A total of 27 (32.1%) patients were ineligible for ICU treatment owing to a high sepsis-related organ failure assessment (SOFA) score [12]; these patients received palliative care.
Figures 1 and 2 respectively depict progression-free survival (PFS) and overall survival (OS) measured from the time of diagnosis in the 84 patients who died from infectious complications. Neither PFS nor OS differed significantly with leukemia type. Median PFS (interquartile range (IQR)) was 0.73 years (0.15 - 1.28) among patients with ALL, 0.35 years (0.08 - 0.80) among patients with AML and 0.15 years (0.05 - 1.00) among the remaining 11 patients. Respective median OS (IQR) was 0.77 years (0.23 - 1.42), 0.44 years (0.08 - 1.04) and 0.15 years (0.05 - 1.01).
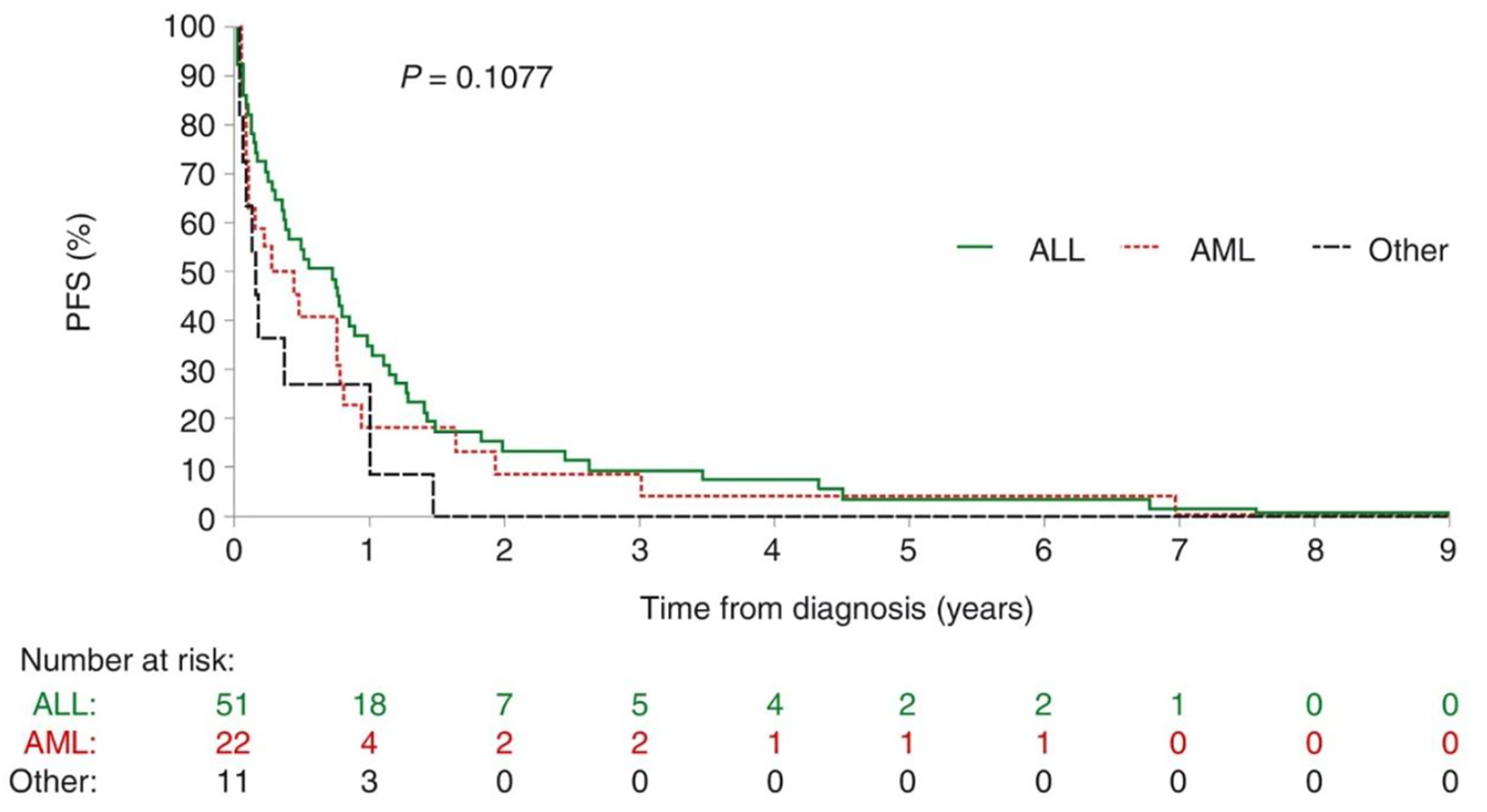 Click for large image | Figure 1. Progression-free survival in patients with acute leukemia who died of infectious complications. There is no difference between the types of leukemias. ALL: acute lymphoblastic leukemia; AML: acute myeloblastic leukemia; PFS: progression-free survival. |
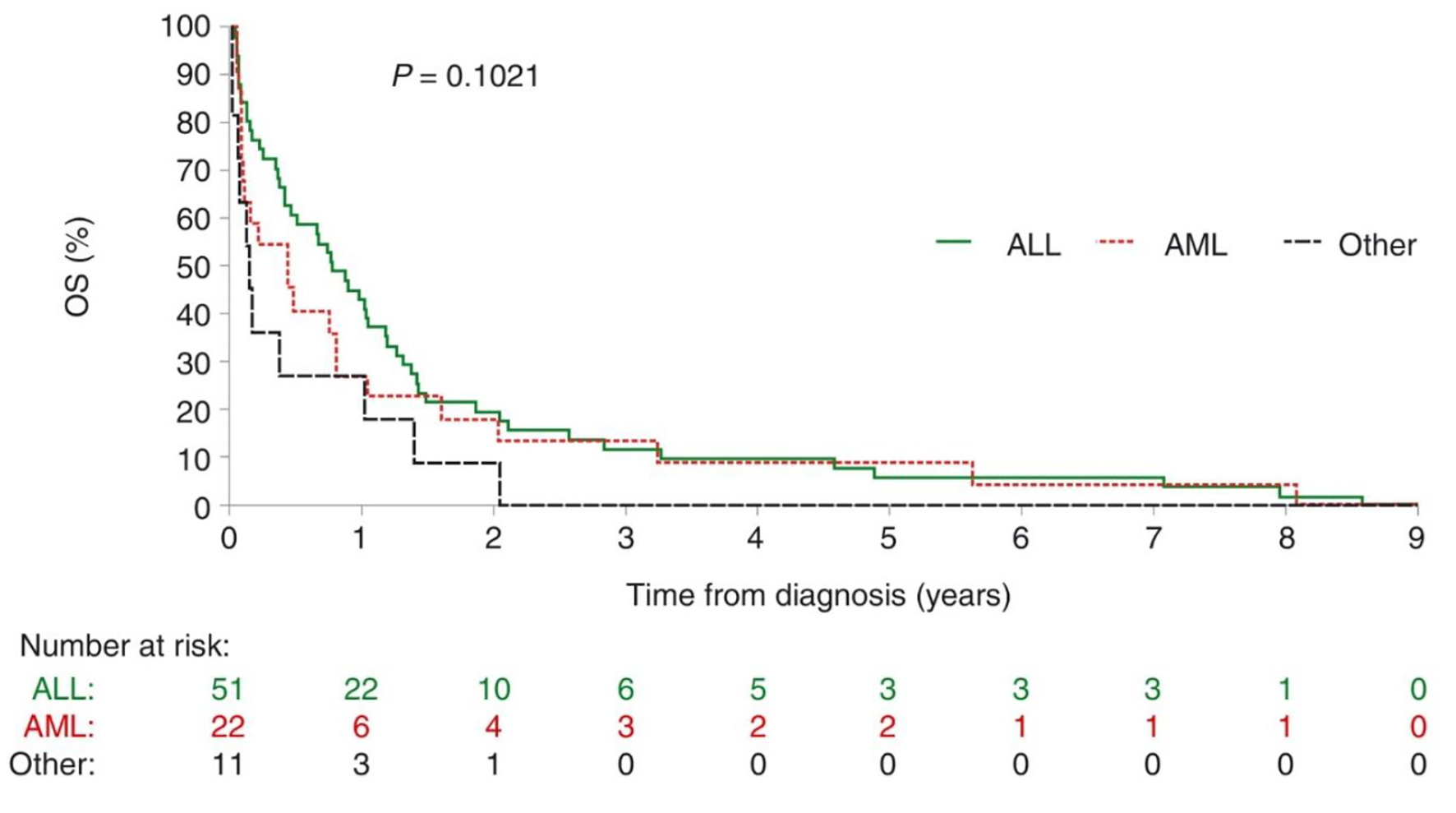 Click for large image | Figure 2. Overall survival in patients with acute leukemia who died of infectious complications. There is no statistical difference between the two types of leukemias, no difference between relapse or naive leukemia in this Kaplan-Meier curves. A division should be made. ALL: acute lymphoblastic leukemia; AML: acute myeloblastic leukemia; OS: overall survival. |
| Discussion | ▴Top |
This retrospective analysis of patients treated in the INCan Acute Leukemia Clinic provides important information regarding rates of treatment-related mortality owing to infectious complications in our patients, and the most common sites of infection and causative organisms. It also highlights which stages of treatment and chemotherapy regimens are most commonly associated with fatal infectious complications. This information will enable us to improve the management of infectious complications in patients with acute leukemia, and could potentially guide treatment selection for patients at increased risk of such complications. The leukemia subgroup analyses are particularly valuable in this respect, as they provide important disease-specific information that can be used to tailor infection management strategies.
Our findings underline that infectious complications are a major problem for patients with hematologic malignancies, including acute leukemias [6, 13-16]. We report a 27% rate of treatment-related mortality owing to infectious complications, which appears similar to, or slightly higher than, that in other recently published reports (13.2-24.7% [17-24]). It should be noted, however, that the timeframe for reporting was not consistent between these analyses, potentially resulting in studies with shorter timeframes reporting lower mortality rates, such as 21-day mortality rates [22]. Among the 84 patients in our analysis who died owing to infectious complications, septic shock was the cause of death in 89%, which is consistent with the high sepsis-associated mortality rates previously reported in patients with hematologic malignancies [14]. The lung was by far the most common primary site of infection (67% of patients), followed by the skin (9%) and the gastrointestinal tract (8%), the latter possibly as a result of microbial translocation associated with the receipt of chemotherapy [25]. Catheter-related bacteremia accounted for 7% of cases, central venous catheters being a recognized potential route for the acquisition of bloodstream infections [26].
We identified a range of different causative organisms, of which gram-negative bacilli were the most common; 12 cases of drug-resistant ESBL-producing E. coli were reported. These findings reflect data highlighted in recent reviews and case reports, which show that E. coli is the most common gram-negative pathogen (comprising a median of 21% of cases in one report) in neutropenic hematology-oncology patients with bloodstream infections [4, 13, 22, 27-30]. The predominance of gram-negative organisms in our analysis is also consistent with other studies and single-center retrospective reports on infectious morbidity and mortality in high-risk hematology patients [2, 4, 20, 22-24, 28-35]. Although the prevalence of fungal infections is increasing, along with the list of fungi that may cause disease [36], we noted only seven deaths owing to fungal infectious complications in our series, allowing to either Candida or Aspergillus. Nevertheless, fungal infections are known to be a major cause of morbidity and mortality in patients with acute leukemia [37, 38], hence almost all the patients in our study who developed febrile neutropenia received antifungal treatment (voriconazole).
We used a combination of blood, urine, feces and/or bronchoscopy culture to identify infectious organisms. In line with international guidelines on the use of antimicrobial agents in cancer patients with neutropenia [39], we initiated broad-spectrum antibiotic therapy in all patients presenting with fever before switching to specific antibiotic therapy upon identification of the infectious agent. Patients with hypotension were treated with meropenem/vancomycin; all others received ceftazidime/amikacin. Our analysis did not take into account levels of patient adherence to the prescribed antibiotic regimen; this has been shown to impact mortality among hospitalized cancer patients with febrile neutropenia, and thus warrants further attention [40]. At INCan, we do not use routinely administered antibiotic prophylaxis to patients without febrile neutropenia, owing to growing concerns regarding drug-resistant microorganisms; however, the value of such prophylaxis, including the potential benefit of cyclical antibiotic prophylaxis for lowering multidrug resistance [41, 42], remains an area of debate, and its utility may depend on local epidemiology and resistance patterns [43, 44]. Similarly, antiviral and/or antifungal prophylaxis is not given routinely in the absence of febrile neutropenia, although recent guidelines from the German Society for Hematology and Medical Oncology recommend primary prophylaxis for invasive fungal infections in patients with hematologic malignancies [45]. Our findings suggest that prophylactic therapy against the most common infectious organisms could be valuable, and highlight the suboptimal utility of broad-spectrum antibiotics in the cases of some infectious organisms, including resistant strains such as ESBL-producing E. coli. They also highlight the impact of financial concerns on treatment choices made by our patient population: over a quarter of the patients who died owing to infectious complications chose not to receive ICU treatment because of financial constraints.
The risk of infectious complications associated with neutropenia/febrile neutropenia in patients with acute leukemia varies depending on the myelosuppressive nature of the chemotherapy regimen employed [3]. In our retrospective analysis, the most common regimen received within 45 days prior to death from infectious complications was hyper-CVAD, an intensive regimen that is associated with high rates of neutropenia [46, 47]. While the use of prophylactic colony-stimulating factors can reduce the incidence of infection in neutropenic patients [6], this does not eliminate the risk completely, as demonstrated by our findings: 59 patients died owing to infectious complications during an episode of febrile neutropenia, despite receiving granulocyte colony-stimulating factor treatment. The link between regimen intensity and infection risk is further underlined by the fact that almost a third of all deaths from infectious complications in our population occurred in patients who were undergoing induction therapy, with a further 30% being in patients receiving treatment for a first relapse. By contrast, only two deaths associated with infectious complications occurred in patients who were receiving low-dose maintenance regimens, and only one patient died of infectious complications while receiving palliative care. These findings are consistent with the observations of Keng and Sekeres, who noted that, while febrile neutropenia can occur at any point during the course of malignancy, most cases develop during initial chemotherapy [47]. Importantly, however, our data also demonstrate that patients with acute leukemia can develop infectious complications several years after diagnosis. This suggests that patients experience prolonged immunosuppression or treatment-related toxicity, or become susceptible to infections owing to the long-term effects of their disease, indicating a need for long-term management and supportive care.
Although our analysis has a number of limitations, including retrospective data collection, extensive data collection for only a limited subset of patients and the fact that all the patients were from a single center, the results obtained nevertheless provide important information regarding the infectious complications experienced by patients with acute leukemia. These findings will help inform the INCan infection control program by increasing knowledge of the local epidemiology and resistance patterns [2], and may thereby assist in reducing mortality rates associated with such complications in this setting.
Acknowledgments
The authors would like to thank the fellows and nurses of the INCan Hematology and Infectious Diseases Departments for the care given to the patients daily. Medical writing support in the preparation of this manuscript was provided by Steve Hill PhD and Sandralee Lewis PhD on behalf of the Investigator Initiated Research Writing Group (an initiative from Ashfield Healthcare, a part of UDG Healthcare plc). The results reported in this manuscript were presented in part at 57 Congreso Nacional de la Agrupacion Mexicana para el Estudio de la Hematologia, Merida, Mexico, April 27 to May 1, 2016, and at the 57th American Society of Hematology Annual Meeting, Orlando, Florida, USA, on December 2 - 5, 2015.
Financial Support
Professional medical writing support in the preparation of this manuscript was financed by Celgene.
Conflict of Interest
The authors have no potential conflict of interest to disclose.
Informed Consent
Written informed consent was obtained to all patients.
Author Contributions
Jorge Torres-Flores: fulfill database; Ramiro Espinoza-Zamora: fulfill database; Jorge Garcia-Mendez: review information; Eduardo Cervera-Ceballos: review information; Alejandro Sosa-Espinoza: study coordinator; Nidia Zapata-Canto: writing and analysis of the database.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Sive JI, Buck G, Fielding A, Lazarus HM, Litzow MR, Luger S, Marks DI, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol. 2012;157(4):463-471.
doi pubmed - Gustinetti G, Mikulska M. Bloodstream infections in neutropenic cancer patients: A practical update. Virulence. 2016;7(3):280-297.
doi pubmed - Antoniadou A, Giamarellou H. Fever of unknown origin in febrile leukopenia. Infect Dis Clin North Am. 2007;21(4):1055-1090, x.
doi pubmed - Kuo FC, Wang SM, Shen CF, Ma YJ, Ho TS, Chen JS, Cheng CN, et al. Bloodstream infections in pediatric patients with acute leukemia: Emphasis on gram-negative bacteria infections. J Microbiol Immunol Infect. 2017;50(4):507-513.
doi pubmed - Klastersky J. Management of fever in neutropenic patients with different risks of complications. Clin Infect Dis. 2004;39(Suppl 1):S32-37.
doi pubmed - Alibek K, Bekmurzayeva A, Mussabekova A, Sultankulov B. Using antimicrobial adjuvant therapy in cancer treatment: a review. Infect Agent Cancer. 2012;7(1):33.
doi pubmed - Baden LR, Swaminathan S, Angarone M, Blouin G, Camins BC, Casper C, Cooper B, et al. Prevention and treatment of cancer-related infections, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(7):882-913.
doi pubmed - United States National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Prevention and Treatment of Cancer-Related Infections, Version 1.2018. www.nccn.org: United States National Comprehensive Cancer Network, 2017.
- Chang JE, Medlin SC, Kahl BS, Longo WL, Williams EC, Lionberger J, Kim K, et al. Augmented and standard Berlin-Frankfurt-Munster chemotherapy for treatment of adult acute lymphoblastic leukemia. Leuk Lymphoma. 2008;49(12):2298-2307.
doi pubmed - Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P, Duggan D, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 1995;85(8):2025-2037.
doi pubmed - Cataland SR, Daugherty CK, Weseman EC, Larson RA. Preliminary experience with a new chemotherapy regimen for adults with acute lymphoblastic leukemia. Leuk Lymphoma. 2001;41(3-4):297-307.
doi pubmed - Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707-710.
doi pubmed - Blennow O, Ljungman P. The challenge of antibiotic resistance in haematology patients. Br J Haematol. 2016;172(4):497-511.
doi pubmed - Cohen J, Drage S. How I manage haematology patients with septic shock. Br J Haematol. 2011;152(4):380-391.
doi pubmed - Giamarellou H, Antoniadou A. Infectious complications of febrile leukopenia. Infect Dis Clin North Am. 2001;15(2):457-482.
doi - Mulanovich V, Kontoyiannis DP. Acute myeloid leukemia and the infectious diseases consultant. Leuk Lymphoma. 2018;59(6):1284-1291.
doi pubmed - Hong J, Woo HS, Ahn HK, Sym SJ, Park J, Cho EK, Shin DB, et al. Pre-treatment blood inflammatory markers as predictors of systemic infection during induction chemotherapy: results of an exploratory study in patients with acute myeloid leukemia. Support Care Cancer. 2016;24(1):187-194.
doi pubmed - Philip C, George B, Ganapule A, Korula A, Jain P, Alex AA, Lakshmi KM, et al. Acute myeloid leukaemia: challenges and real world data from India. Br J Haematol. 2015;170(1):110-117.
doi pubmed - Rosa RG, Goldani LZ. Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob Agents Chemother. 2014;58(7):3799-3803.
doi pubmed - Jacob LA, Lakshmaiah KC, Govindbabu K, Suresh TM, Lokanatha D, Sinha M, Vijaykumar BR, et al. Clinical and microbiological profile of febrile neutropenia in solid tumors and hematological malignancies at a tertiary cancer care center in South India. Indian J Cancer. 2014;51(4):464-468.
doi pubmed - Lakshmaiah KC, Malabagi AS, Govindbabu, Shetty R, Sinha M, Jayashree RS. Febrile neutropenia in hematological malignancies: clinical and microbiological profile and outcome in high risk patients. J Lab Physicians. 2015;7(2):116-120.
doi pubmed - Trecarichi EM, Pagano L, Candoni A, Pastore D, Cattaneo C, Fanci R, Nosari A, et al. Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin Microbiol Infect. 2015;21(4):337-343.
doi pubmed - Gudiol C, Bodro M, Simonetti A, Tubau F, Gonzalez-Barca E, Cisnal M, Domingo-Domenech E, et al. Changing aetiology, clinical features, antimicrobial resistance, and outcomes of bloodstream infection in neutropenic cancer patients. Clin Microbiol Infect. 2013;19(5):474-479.
doi pubmed - Wang L, Wang Y, Fan X, Tang W, Hu J. Prevalence of Resistant Gram-Negative Bacilli in Bloodstream Infection in Febrile Neutropenia Patients Undergoing Hematopoietic Stem Cell Transplantation: A Single Center Retrospective Cohort Study. Medicine (Baltimore). 2015;94(45):e1931.
doi pubmed - Ellis M. Preventing microbial translocation in haematological malignancy. Br J Haematol. 2004;125(3):282-293.
doi pubmed - Greene JN. Catheter-related complications of cancer therapy. Infect Dis Clin North Am. 1996;10(2):255-295.
doi - Ramphal R. Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin Infect Dis. 2004;39(Suppl 1):S25-31.
doi pubmed - Kara O, Zarakolu P, Ascioglu S, Etgul S, Uz B, Buyukasik Y, Akova M. Epidemiology and emerging resistance in bacterial bloodstream infections in patients with hematologic malignancies. Infect Dis (Lond). 2015;47(10):686-693.
doi pubmed - Chen CY, Tang JL, Hsueh PR, Yao M, Huang SY, Chen YC, Chen YC, et al. Trends and antimicrobial resistance of pathogens causing bloodstream infections among febrile neutropenic adults with hematological malignancy. J Formos Med Assoc. 2004;103(7):526-532.
- Sengar M, Kelkar R, Jain H, Biswas S, Pawaskar P, Karpe A. Frequency of bacterial isolates and pattern of antimicrobial resistance in patients with hematological malignancies: A snapshot from tertiary cancer center. Indian J Cancer. 2014;51(4):456-458.
doi pubmed - Conn JR, Catchpoole EM, Runnegar N, Mapp SJ, Markey KA. Low rates of antibiotic resistance and infectious mortality in a cohort of high-risk hematology patients: A single center, retrospective analysis of blood stream infection. PLoS One. 2017;12(5):e0178059.
doi pubmed - Taj M, Farzana T, Shah T, Maqsood S, Ahmed SS, Shamsi TS. Clinical and Microbiological Profile of Pathogens in Febrile Neutropenia in Hematological Malignancies: A Single Center Prospective Analysis. J Oncol. 2015;2015:596504.
doi pubmed - Noronha V, Joshi A, Patil VM, Bhosale B, Muddu VK, Banavali S, Kelkar R, et al. Pattern of infection, therapy, outcome and risk stratification of patients with febrile neutropenia in a tertiary care oncology hospital in India. Indian J Cancer. 2014;51(4):470-474.
doi pubmed - Hummel M, Warga C, Hof H, Hehlmann R, Buchheidt D. Diagnostic yield of blood cultures from antibiotic-naive and antibiotically treated patients with haematological malignancies and high-risk neutropenia. Scand J Infect Dis. 2009;41(9):650-655.
doi pubmed - Siddaiahgari S, Manikyam A, Kumar KA, Rauthan A, Ayyar R. Spectrum of systemic bacterial infections during febrile neutropenia in pediatric oncology patients in tertiary care pediatric center. Indian J Cancer. 2014;51(4):403-405.
doi pubmed - Hsu LY, Ng ES, Koh LP. Common and emerging fungal pulmonary infections. Infect Dis Clin North Am. 2010;24(3):557-577.
doi pubmed - Bhatt VR, Viola GM, Ferrajoli A. Invasive fungal infections in acute leukemia. Ther Adv Hematol. 2011;2(4):231-247.
doi pubmed - Michallet M, Benet T, Sobh M, Kraghel S, El Hamri M, Cannas G, Nicolini FE, et al. Invasive aspergillosis: an important risk factor on the short- and long-term survival of acute myeloid leukemia (AML) patients. Eur J Clin Microbiol Infect Dis. 2012;31(6):991-997.
doi pubmed - Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, Feld R, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34(6):730-751.
doi pubmed - Rosa RG, Goldani LZ, dos Santos RP. Association between adherence to an antimicrobial stewardship program and mortality among hospitalised cancer patients with febrile neutropaenia: a prospective cohort study. BMC Infect Dis. 2014;14:286.
doi pubmed - Teranishi H, Koga Y, Nishio H, Kato W, Ono H, Kanno S, Nakashima K, et al. Clinical efficacy of cycling empirical antibiotic therapy for febrile neutropenia in pediatric cancer patients. J Infect Chemother. 2017;23(7):463-467.
doi pubmed - Chong Y, Shimoda S, Yakushiji H, Ito Y, Miyamoto T, Kamimura T, Shimono N, et al. Antibiotic rotation for febrile neutropenic patients with hematological malignancies: clinical significance of antibiotic heterogeneity. PLoS One. 2013;8(1):e54190.
doi pubmed - Chong Y, Yakushiji H, Ito Y, Kamimura T. Clinical impact of fluoroquinolone prophylaxis in neutropenic patients with hematological malignancies. Int J Infect Dis. 2011;15(4):e277-281.
doi pubmed - Garnica M, Nouer SA, Pellegrino FL, Moreira BM, Maiolino A, Nucci M. Ciprofloxacin prophylaxis in high risk neutropenic patients: effects on outcomes, antimicrobial therapy and resistance. BMC Infect Dis. 2013;13:356.
doi pubmed - Mellinghoff SC, Panse J, Alakel N, Behre G, Buchheidt D, Christopeit M, Hasenkamp J, et al. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann Hematol. 2018;97(2):197-207.
doi pubmed - Gill S, Lane SW, Crawford J, Cull G, Joske D, Marlton P, Mollee PN, et al. Prolonged haematological toxicity from the hyper-CVAD regimen: manifestations, frequency, and natural history in a cohort of 125 consecutive patients. Ann Hematol. 2008;87(9):727-734.
doi pubmed - Kantarjian H, Thomas D, O'Brien S, Cortes J, Giles F, Jeha S, Bueso-Ramos CE, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788-2801.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


