| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Letter to the Editor
Volume 10, Number 1, February 2021, pages 35-39
Similar Outcomes in Early-Failure Steroid-Dependent Compared to Upfront Steroid Refractory Acute Graft-Versus-Host Disease Following Allogeneic Hematopoietic Cell Transplant
Dilan A. Patela, e, Karina A. Mendozab, c, Heidi Chend, Brian G. Engelhardta, Bipin N. Savania, Stacey A. Goodmana, Jeremy Warnera, Adetola A. Kassima, Michael Byrnea, Wichai Chinratanalaba, John P. Greera, Carole Hunta, Madan Jagasiaa
aDepartment of Hematology and Bone Marrow Transplant, Vanderbilt-Ingram Cancer Center, Nashville, TN, USA
bVanderbilt University School of Medicine, Nashville, TN, USA
cInternal Medicine, University of Washington Affiliated Hospitals, Seattle, WA, USA
dDepartment of Biostatistics, Vanderbilt University School of Medicine, Nashville, TN, USA
eCorresponding Author: Dilan A. Patel, Department of Hematology and Bone Marrow Transplant, Vanderbilt-Ingram Cancer Center, Nashville, TN 37232, USA
Manuscript submitted November 4, 2020, accepted November 11, 2020, published online February 6, 2021
Short title: Steroid-Dependent GvHD Outcomes
doi: https://doi.org/10.14740/jh761
| To the Editor | ▴Top |
Acute graft-versus-host disease (aGvHD) remains a barrier to success following allogeneic hematopoietic cell transplant (allo-HCT) [1-5]. Up to 70% of patients are non-responders to first-line systemic corticosteroids and require second-line therapy [2-6]. Non-responders can be further categorized as either upfront steroid refractory (USR), characterized by worsening aGvHD after 3 days of high-dose corticosteroids or with no response after 7 days, or steroid dependent (SD), which is conceptualized as either early-failure (EF-SD) if aGvHD recurs at greater than 50% of the initial corticosteroid dose, or late-failure (LF-SD) if at less than 50% of the starting dose [7-10]. Outcomes for SD patients are not as well studied compared to those with USR disease. Currently, patients with EF-SD or LF-SD aGvHD are not eligible for clinical trials with novel agents either in combination with high-dose corticosteroids or alone based on current criteria [9]. Accordingly, patients must be re-challenged with higher corticosteroid doses to fulfill the criteria of steroid refractory aGvHD prior to pursuing clinical trial enrollment. To our knowledge, analysis of outcomes between USR and SD patients has not been conducted before. We hypothesized that patients with EF-SD aGvHD have similar poor overall survival and non-relapse mortality compared to USR patients.
To address this, we conducted a single-institution retrospective study. Patient data were collected as part of a study that was approved by the Institutional Review Board. The patient population consisted of adult patients 18 years of age or older who underwent allo-HCT between January 2005 and December 2013, and received at least 1 mg/kg prednisone equivalent for aGvHD. Transplant platforms included human leukocyte antigen-matched sibling donors, matched unrelated donors, and double umbilical cord blood grafts. We included patients who received GvHD prophylaxis with calcineurin inhibitors plus methotrexate or mycophenolate mofetil and received systemic corticosteroids for aGvHD. Only patients who underwent matched unrelated donor or mismatched unrelated donor transplants received anti-thymocyte globulin. The diagnosis of chronic GvHD was made using the National Institutes of Health (NIH) consortium criteria after features were identified by the provider [11]. We excluded patients with primary disease relapse or progression before aGvHD or who underwent more than one allo-HCT. Patients were followed until last contact or death. Primary outcomes were overall survival and non-relapse mortality at 1 and 2 years after starting corticosteroids. Secondary outcomes include day 28 response, use of second-line aGvHD therapy, incidence of chronic GvHD, and cause of death.
Patient baseline characteristics were summarized using descriptive statistics, which included the median and interquartile range as well as percentages and frequencies for categorical parameters presented. For the group comparisons, the Wilcoxon rank-sum test was used for comparison of continuous variables and Chi-squared test for categorical variables. Time-dependent proportional hazards methods were used to assess all survival endpoints associated with EF-SD and LF-SD. Time-dependent methods were not used for analysis of survival endpoints associated with USR, as by definition, the onset of up-front corticosteroid-resistance occurred within 10 days after starting corticosteroid treatment. Non-relapse mortality was computed using a competing risk methodology, with relapse as a competing risk. All survival results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs) and P values. Cumulative incidence curves were created using the Fine-Gray method. All P values are reported as two-sided and all analyses were conducted using R 3.5.1 software.
One hundred patients were evaluated. Baseline demographic and transplant-related data are listed (Table 1). No statistically significant differences in baseline characteristics were found between groups. In terms of primary outcome measures, the 1-year (44% vs. 62%) and 2-year overall survival (42% vs. 45%) did not differ significantly between USR and SD groups, respectively. The median overall survival (in years) for USR, EF-SD, and LF-SD was 0.43 (95% CI: 0.28 - 3.97), 1.20 (95% CI: 0.72-5.92% and 2.26 (95% CI: 1.31 - not reached), respectively (Fig. 1). The 1-year and 2-year overall survival was 54% and 33% for EF-SD and 67% and 53% for LF-SD, respectively. Univariate analyses (Cox regression time-dependent) showed that patients in the EF-SD subset (HR: 0.96, 95% CI: 0.54 - 1.71, P = 0.88) did not have a significantly different survival compared to USR (reference category). Patients in the LF-SD subset had an improved survival (HR: 0.54, 95% CI: 0.31 - 9.95, P = 0.03) compared to USR (reference category). With LF-SD as a reference category, the USR subset had an inferior outcome (HR: 1.85, 95% CI: 1.05 - 3.25, P = 0.03), while EF-SD did not show a significant difference (HR: 1.76, 95% CI: 0.94 - 3.32, P = 0.078).
 Click to view | Table 1. Baseline Demographics Stratified by Acute GvHD Subtype |
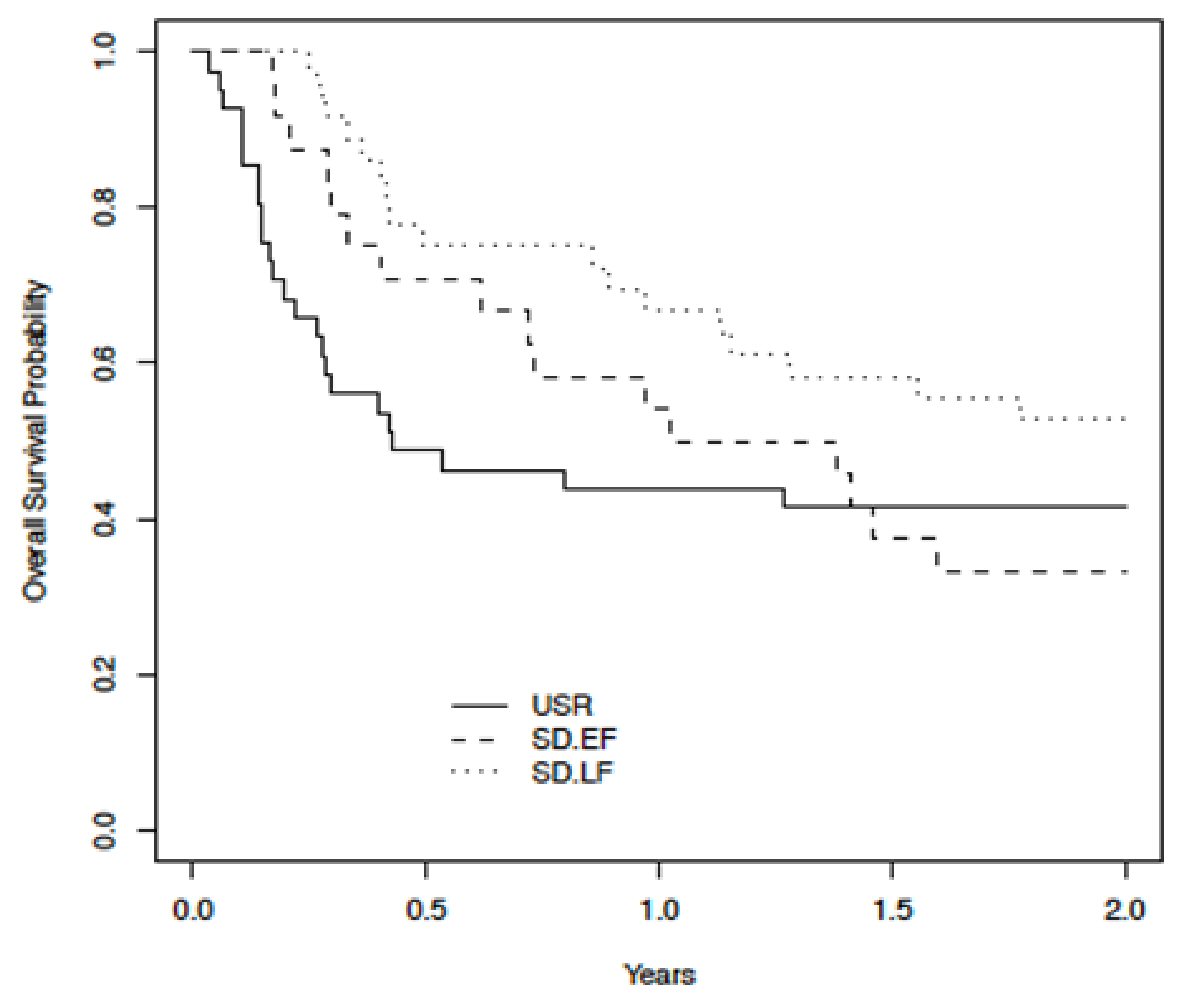 Click for large image | Figure 1. Kaplan-Meier plot (time-dependent Cox regression univariate analysis) comparing overall survival across USR, EF-SD, and LF-SD. USR: upfront steroid refractory; EF-SD: early-failure steroid-dependent; LF-SD: late-failure steroid-dependent. |
Multivariate time-dependent analysis showed that both USR (HR: 1.88, 95% CI: 1.04 - 3.38, P = 0.037) and EF-SD (HR: 1.99, 95% CI: 1.05 - 3.79, P = 0.036) were independent predictors of inferior overall survival. Overall survival was similar for USR compared to EF-SD.
Multivariable analyses (Cox regression time-dependent), adjusted for starting dose of prednisone at onset of aGvHD, age, donor type, and regimen intensity, using USR as reference, showed LF-SD was independently associated with improved overall survival (HR: 0.53, 95% CI: 0.3 - 0.96, P = 0.037). EF-SD had a HR of 1.06 (95% CI: 0.58-1.94%, P = 0.84). Using the same methodology, and adjusted for the same covariates as mentioned above, with LF-SD as reference, both USR (HR: 1.88, 95% CI: 1.04 - 3.38, P = 0.037) and EF-SD (HR: 1.99, 95% CI: 1.05 - 3.79, P = 0.036) were independent predictors of inferior overall survival.
The 1-year and 2-year cumulative incidence of non-relapse mortality, with relapse as competing risk, did not differ significantly among the three groups (P = 0.082) (Fig. 2). The 1-year and 2-year incidence of non-relapse mortality for USR, EF-SD, and LF-SD was (0.42, 95% CI: 0.26 - 0.57), (0.38, 95% CI: 0.18 - 0.58), and (0.19, 95% CI: 0.06 - 0.33), respectively.
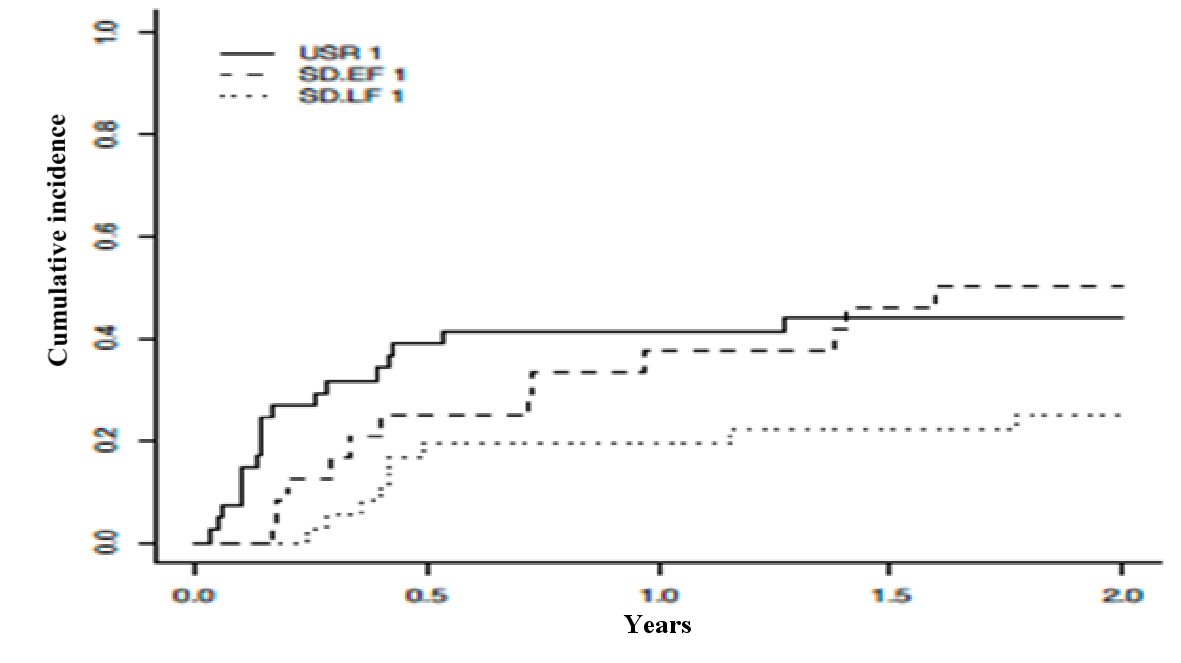 Click for large image | Figure 2. Cumulative incidence of non-relapse mortality with relapse as a competing risk across USR, EF-SD, and LF-SD. USR: upfront steroid refractory; EF-SD: early-failure steroid-dependent; LF-SD: late-failure steroid-dependent. |
In terms of secondary outcome analyses, complete response at day 28 was worse for USR compared to SD disease (20% vs. 43%, P = 0.03), even though the same initial corticosteroid dose of 1.3 mg/kg was used in both groups. The maximum grade of aGvHD did not differ between the groups. In addition, the number of days until aGvHD or chronic GvHD did not differ. USR patients were maintained on high-doses of corticosteroids and were also more likely to receive second-line aGvHD treatment (USR vs. SD; 63% vs. 35%, P = 0.005).
In terms of comparisons between USR and EF-SD, complete response to day 28 high-dose corticosteroids did not significantly differ between patients with USR and EF-SD (20% and 25%, respectively). Similar rates of partial response and stable disease were also found between the groups. The initial dose of corticosteroids in both groups was 1.3 mg/kg. Days until manifestation of aGvHD were similar (26 vs. 20, P = 0.1), though the time to chronic GvHD differed (300 vs. 68, P = 0.008). However, the development of chronic GvHD did not significantly differ (39% vs. 50%, P = 0.4).
For comparisons between EF-SD and LF-SD, the pattern of response to day 28 high-dose corticosteroids was inversely proportional and differed significantly with a 25% vs. 56% complete response, respectively, (P = 0.001). The same starting doses of corticosteroids of 1.3 mg/kg were given. However, EF-SD patients were found to receive higher doses of corticosteroids at disease recurrence (1.1 mg/kg vs. 0.4 mg/kg, P <0.001). Significant differences did not exist for days until acute (20 vs. 22) or chronic GvHD (68 vs. 200) or the development of chronic GvHD (50% vs. 64%) between EF-SD and LF-SD, respectively.
The primary cause of death in patients with USR and EF-SD GvHD was GvHD (64% vs. 47%, respectively). On the other hand, the majority of deaths in LF-SD patients were due to relapse of the underlying hematologic disease, with 43% of deaths attributed to relapse and 20% to GvHD.
These results show that patients with USR and EF-SD aGvHD have similarly poor survival outcomes. As expected, day 28 response was lower in patients with USR compared to EF-SD. Although the grade of aGvHD was not different among these two groups, we speculate that clinical features not represented in the staging and grading of aGvHD contributed to an “unconscious” physician bias of using a higher corticosteroid dose in patients who eventually were USR [10]. While our findings validate the known poor outcomes seen with USR aGvHD, we have identified a subset of patients, those with EF-SD aGvHD, who appear clinically similar. Clinical trials for second-line aGvHD therapy have typically not included SD patients [12, 13].
More recently, the US Food and Drug Administration (FDA) approved ruxolitinib, a JAK1/JAK2 inhibitor, for corticosteroid-refractory aGvHD, based on a single arm, phase 2 clinical trial, REACH-1 [14]. This study appropriately included patients who were taper intolerant, defined as recurrence of aGvHD at a dose higher than 50% of initial dose of corticosteroid [14]. Ruxolitinib is also active for chronic GvHD in the salvage setting, suggesting the importance of extended cytokine blockade for both B-cell and T-cell mediated disease processes [12]. The randomized phase 3 REACH-2 study confirmed the benefit of ruxolitinib in the corticosteroid refractory population [15]. Generalizability of our findings is limited by the retrospective and single center nature of our study along with between group differences in baseline characteristics and heterogeneity in treatment courses. Large collaborative or registry studies are needed to validate outcomes and expand and modernize eligibility criteria for second-line therapy.
Acknowledgments
The authors would like to thank the patients and staff at Vanderbilt University Medical Center for their contributions to this research.
Financial Disclosure
None to declare.
Conflict of Interest
MJ notes consultancy for Incyte, Kadmon, Mallinckrodt. The other authors have no interests to disclose.
Informed Consent
Not applicable.
Author Contributions
DP wrote the manuscript and interpreted data. KM collected data. MJ designed the study and wrote the manuscript. KM, BE, BS, SG, JW, AK, MB, WC, and JG contributed to critical review of the manuscript and participated in the clinical care of the patient cohort. HC performed the statistical analysis. CH contributed to data collection.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443-458.
doi pubmed - Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, Urbano-Ispizua A, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296-307.
doi pubmed - Zeiser R, Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med. 2017;377(22):2167-2179.
doi pubmed - Lee SE, Cho BS, Kim JH, Yoon JH, Shin SH, Yahng SA, Eom KS, et al. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplant. 2013;48(4):587-592.
doi pubmed - Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, Litzow MR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150-1163.
doi pubmed - Mielcarek M, Furlong T, Storer BE, Green ML, McDonald GB, Carpenter PA, Flowers ME, et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica. 2015;100(6):842-848.
doi pubmed - Calmettes C, Vigouroux S, Labopin M, Tabrizi R, Turlure P, Lafarge X, Marit G, et al. Risk factors for steroid-refractory acute graft-versus-host disease after allogeneic stem cell transplantation from matched related or unrelated donors. Biol Blood Marrow Transplant. 2015;21(5):860-865.
doi pubmed - Luft T, Dietrich S, Falk C, Conzelmann M, Hess M, Benner A, Neumann F, et al. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118(6):1685-1692.
doi pubmed - Jagasia M, Zeiser R, Arbushites M, Delaite P, Gadbaw B, Bubnoff NV. Ruxolitinib for the treatment of patients with steroid-refractory GVHD: an introduction to the REACH trials. Immunotherapy. 2018;10(5):391-402.
doi pubmed - Hill L, Saliba R, Chen J, et al. Predictors for Steroid tapering in patients with acute graft-versus-host disease and their impact on graft-versus-host disease response and non-relapse mortality: older age and co-morbidities are associated with quicker tapering and worse outcomes. Blood. 2016;128:3424.
doi - Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401 e381.
- Hill L, Alousi A, Kebriaei P, Mehta R, Rezvani K, Shpall E. New and emerging therapies for acute and chronic graft versus host disease. Ther Adv Hematol. 2018;9(1):21-46.
doi pubmed - Lazaryan A, Lee S, Salhotra A, et al. Initial results of KD025-208: a phase 2a open-label clinical trial of KD025 for steroid-dependent chronic graft versus host disease (cGVHD). Biol Blood Marrow Transplant. 2018;24(3):S70.
doi - Jagasia M, Perales MA, Schroeder MA, Ali H, Shah NN, Chen YB, Fazal S, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739-1749.
doi pubmed - Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, Szer J, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800-1810.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


