| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 10, Number 4, August 2021, pages 178-186
Azithromycin Reduces Markers of Vascular Damage in Pediatric Patients With Sickle Cell Disease
Peter N. Uchakina, b, g, Vishwas S. Sakhalkarc, Francis C. Daned, Olga N. Uchakinaa, Jatayah N. Sheede, William T. Uphousee, Om V. Sakhalkarf
aDepartment of Biomedical Sciences, Mercer University School of Medicine, Macon, GA 31207, USA
bDepartment of Internal Medicine, Mercer University School of Medicine, Macon, GA 31207, USA
cDivision of Pediatric Hematology/Oncology, Mercer University School of Medicine, Beverly Knight Olson Children’s Hospital and HOPE Clinic at Atrium Health-Navicent, Macon, GA 31201, USA
dDepartment of Psychology, Radford University, Roanoke, VA 24013, USA
eMercer University School of Medicine, Macon, GA31207, USA
fAugusta University, Augusta, GA, USA
gCorresponding Author: Peter N. Uchakin, Mercer University School of Medicine, 1550 College Street, Macon, GA 31207, USA
Manuscript submitted March 26, 2021, accepted June 16, 2021, published online July 28, 2021
Short title: Use of Azithromycin in Sickle Cell Anemia
doi: https://doi.org/10.14740/jh827
| Abstract | ▴Top |
Background: Immunomodulatory effects of macrolides in chronic inflammation are well known. In this study, we tested our hypothesis that azithromycin (AZT) can decrease inflammation in pediatric patients with sickle cell disease (SCD).
Methods: The use of AZT as an anti-inflammatory agent was evaluated in double-blind, placebo-controlled, cross-over study for 8 weeks of treatment with 8 weeks of washout. Blood samples were collected before (PRE) and after (POST) each 8-week treatment period. Repeated measures analysis of variance (ANOVA) with post hoc multiple comparison procedures and Chi-square test were used for statistical analysis of the data. Complete blood count, distribution of the lymphocyte subsets, and plasma levels of markers of vascular damage were analyzed.
Results: A significant decrease in the number of leucocytes and granulocytes was observed in AZT group following treatment. An opposite dynamic was observed in placebo group; numbers of granulocytes significantly increased at POST interval. All markers of vascular damage were reduced in AZT group at POST interval with overall significance (P = 0.026). The most prominent significant changes were observed in levels of myeloid-related protein 8/14 (MRP8/14), lipocalin A (NGAL), matrix metalloproteinases (MMP) 9, and insulin-like growth factor-binding protein (IGFBP) 4. Plasma level of C-reactive protein (CRP) was significantly decreased in AZT group as well.
Conclusions: Data suggested that AZT may be beneficial in management of microvascular injury in SCD.
Keywords: Inflammation; Vascular damage; Antibiotic; Macrolide
| Introduction | ▴Top |
Sickle cell disease (SCD) is the world’s most common hemoglobinopathy that perhaps originated as nature’s defense against malaria. SCD is a spectrum condition that caused by the single mutation (GAG→GTG and CTC→CAC) in the beta-globin locus on chromosome 11. Results of such mutation lead to production of the defective hemoglobin S, which polymerizes following its deoxygenation and sickling of erythrocytes. Change in the shape of the erythrocytes results in the loss of their elasticity, and thus, increases risk of occlusion in the microvasculature, as well as their hemolysis with release of free hemoglobin, which acts as scavenger of nitric oxide. Sickling is also associated with increased dehydration of erythrocytes, decreased antioxidant capacity, elevated exposure of phosphatidylserine, formation of microparticles, which may stimulate coagulation cascade [1-7].
Hydroxyurea (HU) is the only approved medication to ameliorate SCD until recently and the only well studied drug for treatment of SCD. However, it is important to take into consideration that HU is a chemotherapeutic agent, has extensive side effects and is often associated with mucosal and cutaneous ulcerations [8-10], which may also expose tissues to a number of opportunistic pathogens.
Intense systemic inflammation from intravascular hemolysis and vaso-occlusions may lead to many acute and chronic complications such as acute pain episodes, stroke, acute chest syndrome (ACS) and predispose patients to a variety of pulmonary complications. While routine bacterial infections can be treated with third-generation cephalosporins (cefotaxime or ceftriaxone), macrolides (azithromycin (AZT) or erythromycin) are recommended for coverage of atypical microbes in the patients with SCD [11]. Although bacteriostatic and bactericidal properties of macrolides are well established, their ability to modulate host immune response is less known. Anti-inflammatory properties of the macrolides and AZT have been shown before [12-16]. Furthermore, the antiviral properties of AZT have been demonstrated in vitro as well [17, 18]. The exact mechanisms of its actions remain unclear. In this study, we tested our hypothesis that AZT can lower chronic vascular inflammation seen in pediatric patients with SCD.
| Materials and Methods | ▴Top |
Subjects
The anti-inflammatory properties of AZT were evaluated in a 24-week double-blind crossover, placebo-controlled study. Inclusion criteria were: age 6 - 18 years who are able to swallow pills, diagnosis of SCD (HbSS, HbSC, HbSβ0 thalassemia) by hemoglobin electrophoresis, and steady-state health with no history of any acute illness in the past 1 month. Exclusion criteria were: hospital admission in the past 3 months, vaso-occlusive crisis in the past 3 months, history of blood transfusion in the past 3 months, and receiving AZT in the past 2 months.
Ethical issues and informed consent
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. The Navicent Health Institutional Review Board approved the study. Informed consent forms were given and explained to the parents or legal guardians of the children prior to enrollment into the study.
Experimental procedure
One 250 mg capsule of AZT or placebo was given orally for 8 weeks to children under 30 kg and two capsules were given to children over 30 kg thrice weekly, then switched following 8 weeks washout. Subjects did not receive any other medication other than folic acid and standard HU therapy.
Peripheral blood was collected at four intervals: before (PRE) and after (POST) each 8-week AZT or placebo treatment. Twenty pediatric patients (age (mean ± standard deviation (SD)): 12.1 ± 2.9 years) of both genders completed the study.
All blood samples were collected at the physician office during scheduled visits via venipuncture. Complete blood count (CBC), plasma level of C-reactive protein (CRP) and blood chemistry were performed at the clinical laboratory at Navicent Health Medical Center. Samples designated for immune analyses were placed on ice immediately upon collection in the outpatient clinic, and transferred to the research laboratory within 60 min for subsequent assessment.
Assays
White blood cells
Whole blood was used to determine the distribution of white blood cells subsets with flow cytometry. Lymphocyte phenotyping was accomplished by direct immunofluorescence labeling of cell surface antigens with mouse anti-human monoclonal antibodies conjugated to different (peridinin chlorophyll protein (PerCP)/cyanine 5.5 (Cy5.5), fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), PE/Cy7, APC/Cy7) fluorochromes using procedures recommended by the manufacturer (BioLegend, San Diego, CA, USA). Along with physical parameters (forward scatter/side scatter (FS/SS)) PerCP labeled anti-cluster of differentiation (CD)45 antibodies (Abs) were used for leukocyte gating of granulocytes, monocytes, lymphocytes, as well as lymphocyte subsets T cells (CD3+), T helper cells (CD3+CD4+), T cytotoxic cells (CD3+CD8+), T natural killer (CD3-CD56+), and natural killer (NK) cells (CD3-CD56+). Samples were analyzed on a BD FACSCalibur cell analyzer (Beckman Coulter, Brea, CA, USA).
Markers of the vascular damage (VD)
The remainder of the whole blood samples were centrifuged, plasma removed, and then frozen at -80 °C until subsequent batch analysis of the systemic level of the markers of VD. Concentrations of the myoglobin (Myo), myeloid-related protein 8/14 (MRP8/14), lipocalin A (NGAL), matrix metalloproteinases (MMP) 2 and 9, osteopontin (OPN), myeloperoxidase (MPO), serum amyloid A (SAA), insulin-like growth factor-binding protein (IGFBP) 4, intercellular adhesion molecule (ICAM) 1, vascular cell adhesion molecule (VCAM) 1, and cystatin C (Cys) were measured by flow cytometry using LEGENDplex bead-based immunoassay (BioLegend, Ssan Diego CA, USA). Acquired raw data were analyzed using LEGENDplex Data Analysis Software v.7 (VigeneTech, Carlisle, MA, USA).
Statistics
Data from the AZT-treated (AZT) and placebo control (CTRL) groups at each 2-month trial were combined following study completion for statistical analysis. All statistical analyses were accomplished with IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY., USA), SigmaPlot version 13 (Systat Software Inc., San Jose, CA, USA) and Jamovi version 1.6.23 (The jamovi project, Sydney, Australia). Mixed-model repeated measures analysis of variance (ANOVA) was used after determining that distributions conformed to the requirements of normality and homogeneity. Appropriate post hoc multiple comparison procedures (Student-Newman-Keuls Method) were used for statistical analysis of the collected data. For the assessment of relative interval-dependent (percent to PRE) dynamics, absolute units of measure were normalized with log10 and square root transformation. Continuous measures were expressed as means ± standard error of the mean (SEM). Differences between categorical data were analyzed with the Chi-square (χ2) test.
| Results | ▴Top |
There were no significant differences between groups in age (P = 0.978), gender (P = 0.361), and phenotype of the SCD (P = 0.499, Table 1 and Supplementary Material 1, www.thejh.org). No signs of infections were observed or reported during the duration of the study.
 Click to view | Table 1. Demographic Data of the Subjects |
A significant (P = 0.023) decrease in the absolute count of leukocytes was observed in patients of AZT group at POST interval. In contrast, absolute number of leucocytes in peripheral blood of CTRL group significantly (P = 0.007) increased at POST interval compared to PRE interval. Those changes reflected a significant decrease in the percentage and absolute numbers (P = 0.031) of neutrophils in AZT group and their significant (P = 0.034; P = 0.04 correspondingly) increase in CTRL group at POST interval. Such changes were associated with a significant (P = 0.04) increase in percentage of lymphocytes in AZT group. A decrease in this parameter in CTRL group was registered but did not reach statistical significance. Slight but significant (P = 0.006) decrease in the percentage of monocytes was observed in CTRL group. No significant changes in AZT group were registered for this parameter (Table 2).
 Click to view | Table 2. Relative Distribution and Absolute Numbers of the Major Populations of White Blood Cells in Peripheral Blood in Patients Treated With Azithromycin (AZT) and Placebo Control (CTRL) Groups |
No significant changes were observed in the number of erythrocytes.
Subsequently, we evaluated the distribution of the major lymphocyte subsets in plasma of the patients. Percentage of pan T cells (CD3+) and their major subsets of helper (CD3+CD4+ Th) and cytotoxic (CD3+CD8+ Tc) T cells was significantly (P = 0.033; P = 0.016 correspondingly) higher in AZT group following the treatment. However, readjustment to the total number of lymphocytes showed increase in the absolute numbers of pan CD3+ and T helper subset was higher in CTRL group at POST interval. No changes were observed in the distribution of the NK (CD3-CD56+) and TNK (CD3+56+) cells in either group (Table 3).
 Click to view | Table 3. Relative Distribution of the T Cell Subsets in Peripheral Blood of Patients Treated With Azithromycin (AZT) and Placebo Control (CTRL) Groups |
These changes were accompanied by a significant (P = 0.022) decrease in the plasma level of CRP in AZT group (0.33 ± 0.07 vs. 0.18 ± 0.04 mg/L). No significant changes (about 4% decrease) were registered in the lactate dehydrogenase (LDL) level in AZT group.
Plasma levels of all studied parameters of VD, were decreased in AZT group at different degree with overall significance (P = 0.026). A 2 (group) × 2 (time: PRE vs. POST) × 12 (VD marker) mixed-model analysis of variance (ANOVA) was used to determine: 1) if there was a change between studied intervals; 2) if that change was consistent between the AZT and control groups; and 3) if that change was consistent for the different markers. Data were presented as percent difference between PRE and POST intervals (Fig. 1). Specifically, significantly lower plasma levels of MRP8/14, NGAL, SAA, IGFBR4, and MMP9 were observed in AZT group (Fig. 2).
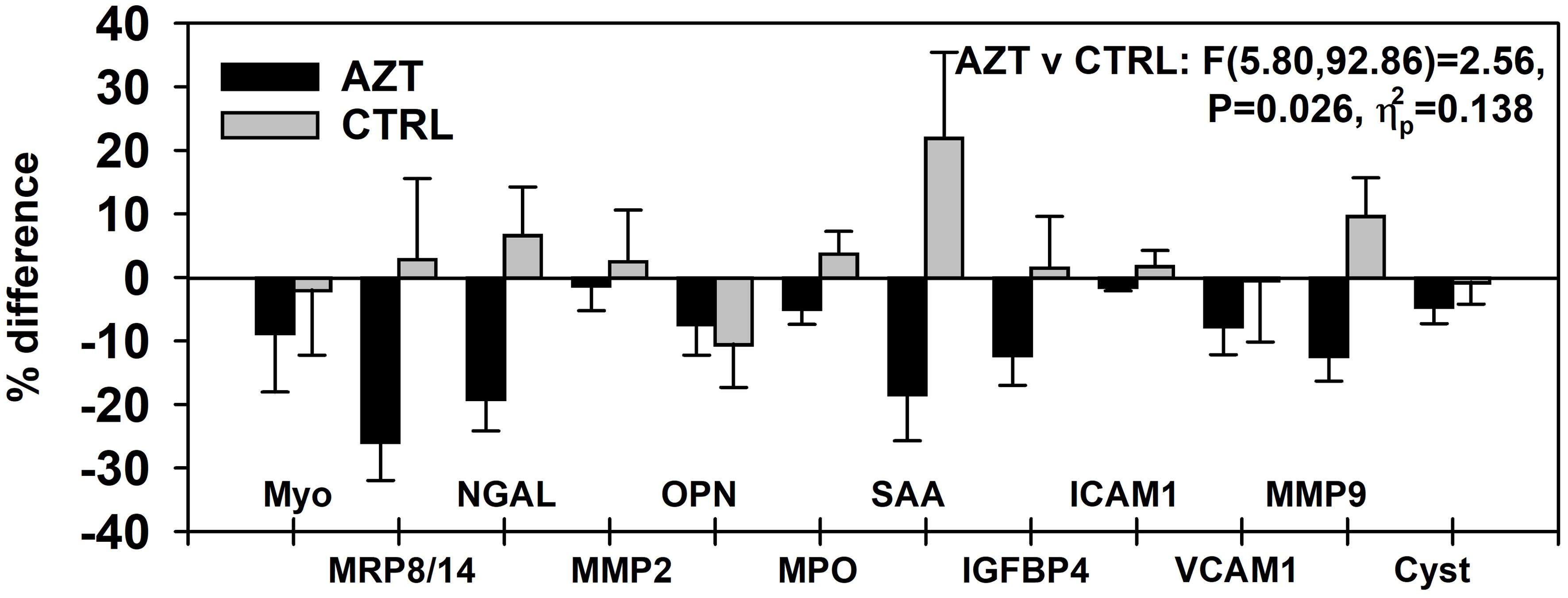 Click for large image | Figure 1. Dynamics of changes in plasma levels of all studied markers of vascular damage in patients treated with azithromycin (AZT) and placebo control (CTRL) groups. Data were analyzed with mixed-model repeated measures analysis of variance (ANOVA) and presented as a percent difference between PRE and POST intervals. MRP: myeloid-related protein; NGAL: lipocalin A; MMP: matrix metalloproteinase; IGFBP: insulin-like growth factor-binding protein; OPN: osteopontin; MPO: myeloperoxidase; SAA: serum amyloid A; ICAM: intercellular adhesion molecule; VCAM: vascular cell adhesion molecule; Cyst: cystatin. |
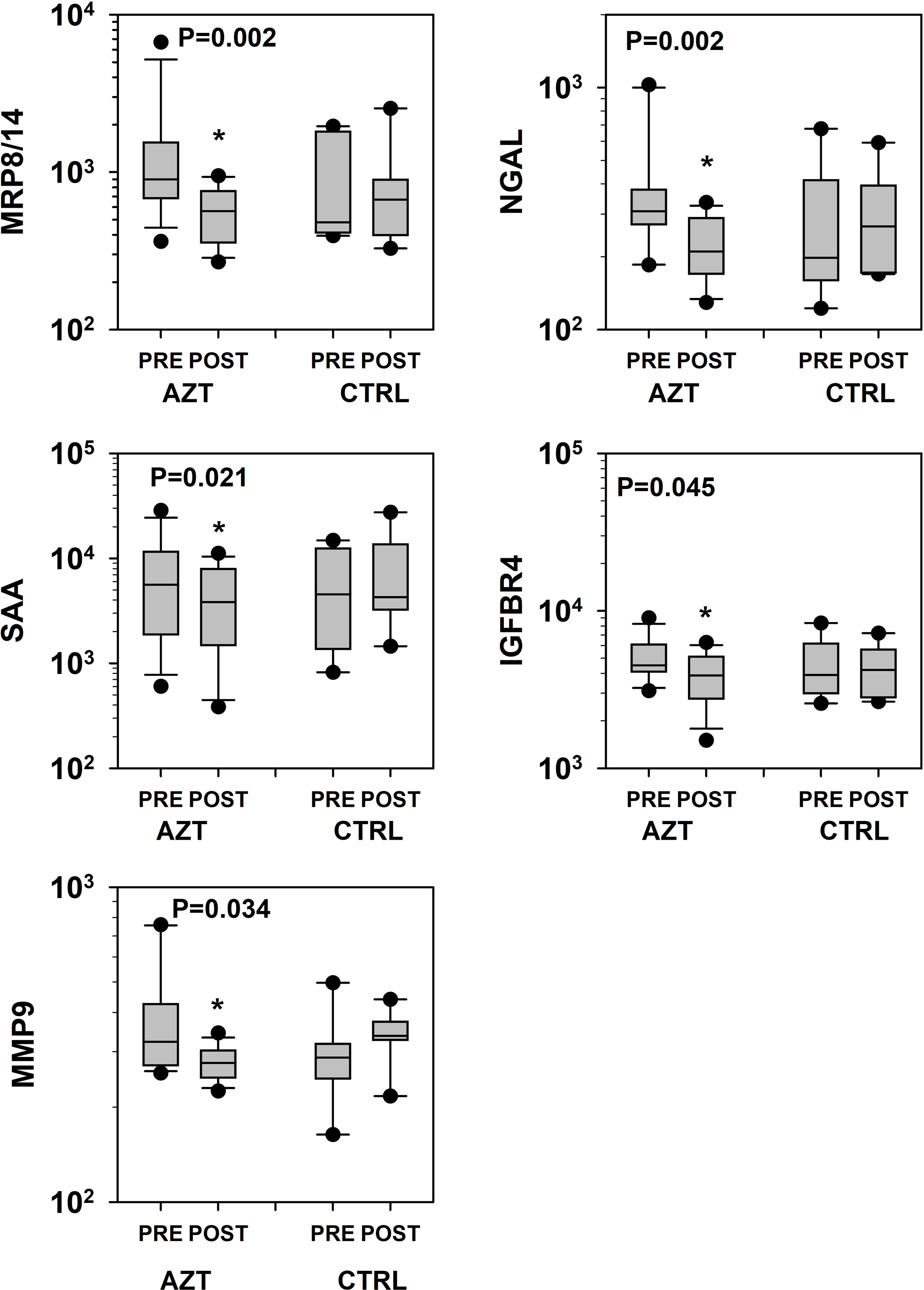 Click for large image | Figure 2. Dynamics of changes in plasma levels of specific markers of vascular damage that revealed statistical significance in patients treated with azithromycin (AZT) and placebo control (CTRL) groups. Data was analyzed with mixed-model repeated measures analysis of variance (ANOVA) followed by the pairwise multiple comparison procedures (Holm-Sidak method). *Statistical significance between PRE and POST intervals. MRP: myeloid-related protein; NGAL: lipocalin A; MMP: matrix metalloproteinase; IGFBP: insulin-like growth factor-binding protein; SAA: serum amyloid A. |
Subsequently, we evaluated the distribution of patients in each group whose plasma levels of any marker of VD has decreased at POST interval. Data were analyzed with χ2 test with Yates correction for continuity and presented as percentage of patients with lowered VD markers relative to the total number of the patients in the group. A significantly higher number (91.7%) of patients in AZT group compared to CTRL group had decreased levels of MRP8/14, with the next highest numbers being 83.3% for the SAA and MMP9, and 75% for NGAL (Fig. 3). Further analysis showed that overall the AZT group included significantly (P = 0.003) more patients with decreased levels of VD markers compared to the CTRL group.
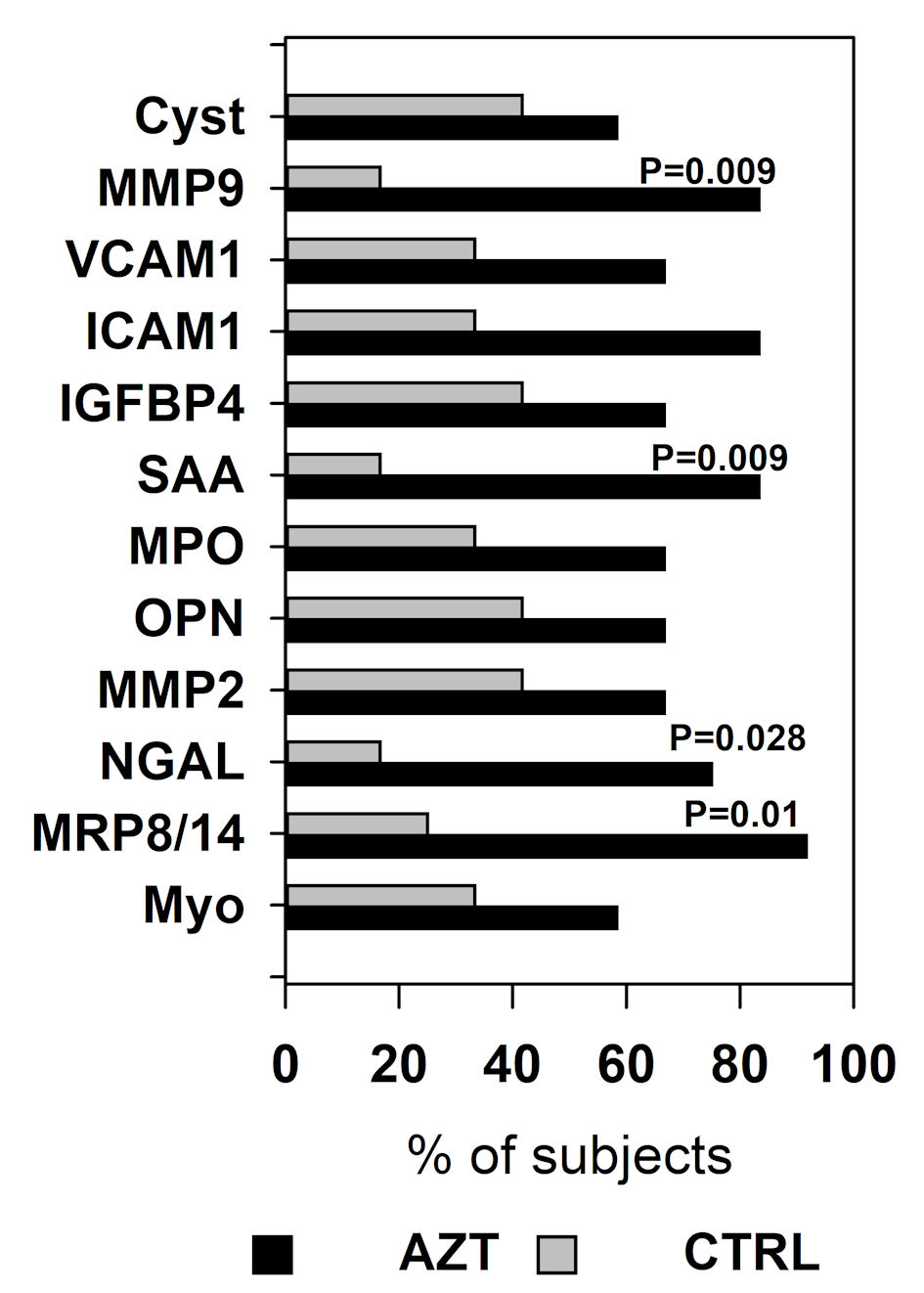 Click for large image | Figure 3. Percentage of the patients in azithromycin (AZT) and placebo control (CTRL) groups whose plasma level decreased at POST interval compared to PRE. Data were analyzed with χ2 test with Yates correction for continuity. MRP: myeloid-related protein; NGAL: lipocalin A; MMP: matrix metalloproteinase; IGFBP: insulin-like growth factor-binding protein; OPN: osteopontin; MPO: myeloperoxidase; SAA: serum amyloid A; ICAM: intercellular adhesion molecule; VCAM: vascular cell adhesion molecule; Cyst: cystatin. |
| Discussion | ▴Top |
In this study we evaluated the use of AZT to lower the inflammatory response due to non-infectious etiology viz. intravascular hemolysis, the major factor responsible for inflammatory damage in SCD. While the underlying mechanisms of such properties of AZT and other macrolides are unclear it is important to accumulate sufficient information for future research. It will allow for evaluation of possible cellular and molecular mechanisms for the treatment of many diseases that are often associated with and cause significant and sometimes fatal yet preventable damage due to systemic inflammation. Understanding these mechanisms might also help improve treatment of inflammation-mediated illnesses. Our results do not definitively explain exact mechanisms of AZT action but rather show some of the outcomes of AZT treatment, and thus, will help to narrow down the future direction(s) in this research.
Vascular endothelium plays a significant role in the development of systemic inflammation. In this study, we observed changes in a number of parameters that reflect the systemic inflammation and state of the immune system.
Despite observed statistical significance in the distribution of the T cell subsets, they are rather small, and their clinical significance should be interpreted very conservatively.
The most apparent and clinically significant finding of this study is that AZT use leads to a decrease in the number of neutrophils, decrease in levels of several markers of VD: CRP, MRP8/14, NGAL, SAA, IGFBP4, and MMP9. All those markers of systemic inflammation are well known. They are released by the tissues as well as by the circulating and resident leucocytes.
The role of studied markers in the pathogenesis of the VD has been demonstrated previously [19-24]. Endothelial inflammation and damage due to intravascular hemolysis result in the activation of inflammatory cascade and release of inflammatory markers, leading to a vicious cycle that causes several pathophysiological manifestations of SCD. AZT was studied in context of studying its effect on mitigating this specific mechanism of endothelial and white blood cell inflammatory response. In SCD patients, hemolysis that is associated with hypoxia from the vaso-occlusions may lead to the necrosis and to exposure of the damage-associated molecular patterns (DAMPs) of damaged tissue to the resident phagocytes, which would initiate the tissue repair and wound healing. However, and more importantly, the same damaged tissue will be exposed to the local commensal microbiota. If pathogenic agent is present and/or the immune system is compromised, such damaged tissue presents an opening for the pathogen to spread locally, and in the worst-case scenario, systemically, which may cause bacteremia/fungemia and even sepsis.
Invasion of the pathogen(s) activates local inflammatory response, which leads to secretion of the proinflammatory cytokines such as tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL1β), IL6, and chemokines (e.g., CXC motif chemokine ligand 8 (CXCL8)) for consequent recruitment of neutrophils and then monocytes/macrophages to the site of infection. The very same proinflammatory cytokines TNFα and IL1β were shown to stimulate release of IGFBP4 from the lung fibroblasts in animal model [25]. Its elevated level was also registered in patients with inflammatory bowel disease, and commonly managed by corticosteroids and infliximab [26]. Similarly, a TNFα-induced increase in MMP9 was demonstrated [27, 28]. The critical role of MMP9 in VD was well shown as well. Wilson et al reported elevated levels of MMP1 and MMP9 in plasma in patients with ruptured abdominal aortic aneurysm (AAA). Also, they demonstrated that an elevated level of MMP9 correlated with non-survival from the rupture surgery. Authors concluded that elevated MMP9 may be considered as a survival predictor/indicator for AAA [29].
In our study we registered significant decrease of these inflammatory mediators/markers following use of the AZT.
Another studied marker, MRP8/14, the decrease of which we observed following AZT treatment in our study is produced by activated phagocytes, and its pro-inflammatory role in the innate and adaptive inflammation has been shown [30-38].
Further, both CRP and SAA were identified as reliable markers of early onset of vaso-occlusive crisis or even within the prodromal phase of SCD [39]. Tumblin et al reported that an increase in the circulating level of SAA was registered during acute painful episodes, especially among patients with marked end-organ impairment [40]. It is important to note that an elevated level of SAA was observed in SCD patients who had pulmonary arterial hypertension, which is one of the most significant complications and comorbidity factors of SCD [41]. Later, both CRP and SAA were evaluated as markers of inflammation among patients with different types of viral and bacterial infections, including bacterial pneumonia and severe bacterial sepsis. Lannergard et al showed that an increase in the plasma of both markers are well correlated with both viral and bacterial infections, and that SAA is a more sensitive marker of systemic infection and can be used as a marker of inflammation in a number of viral infections [42].
In 2005, Yip et al saw elevated levels of SAA in the serum of patients with severe acute respiratory syndrome coronavirus (SARS-CoV). Furthermore, authors showed that concentration of SAA was highly correlated with the progression of infection among four patients, whereas a decrease in the circulating level of SAA was associated with progressive recovery. On the other side of the spectrum, a patient had an elevated level of SAA and a high radiographic score. The patient’s SAA level continued to rise over the course of the treatment. An increase in the SAA was parallel to the increase in the occurrence of opportunistic (e.g., Candida sp., cytomegalovirus (CMV), methicillin-resistant Staphylococcus aureus (MRSA)) infections. While subsequent use of antibiotics decreased the level of SAA, its level and radiographic score remained high until the patient’s demise [43].
Even without systemic spread of the pathogen, local foci of inflammation and necrosis may lead to the development of conditions such as disseminated intravascular coagulation (DIC) and progress to further tissue damage due to tissue hypoxia. Such connection is well known and has been described previously [44]. DIC of different severity among the SCD patients is one of the most significant outcomes of the vascular occlusions [45-47]. Another pathological outcome of such inflammation is ACS, which is the leading cause of morbidity and mortality for patients with SCD [48].
The danger of the development of such “vicious cycles” is apparent, as without tight control by the endogenous (immune system) or/and exogenous (pharmacological intervention) mechanisms, they may lead to a chronic inflammation, and if not treated, to a condition known as “cytokine storm”. The clinical importance and outcomes of such condition cannot be overstated as it may lead to many life-threatening states due to multiple organ failure.
Current dataset cannot conclusively explain underlying mechanisms of decreased circulating levels of CRP, MRP8/14, NGAL, SAA, IGFBP4, and MMP9, and role of the AZT in it. It is unclear if: 1) AZT just helps control opportunistic pathogens in the damaged tissues, and thus decrease systemic inflammation; or 2) AZT also has anti-inflammatory properties which protect tissues and thus, prevent spread of pathogen.
Different markers of inflammation have different half-life and different tropism to different tissues, cellular sources, mechanisms of action (e.g., autocrine, paracrine, and/or endocrine), and as a result, will be differently represented in circulation. Thus, it is important to consider that this combination of all studied parameter at the particular interval reflects rather a “snap shot” of quite plastic process, especially among the patients with a number of various comorbidity factors. That suggests that observed changes likely reflect stable immune state of decreased systemic inflammation, and thus have prospective clinical significance to the management of SCD or other systemic inflammatory conditions where damage to the vascular beds may occur.
Our observations and known antibacterial and antiviral properties of AZT have potential to reevaluate its clinical use to mitigate inflammatory reactions, alongside a modest protection from the opportunistic infections.
One of the limitations of this study is the patient compliance. Of the 23 patients, only 17 (about 74% of all subjects) successfully completed the study. Drop-off was due to non-compliance of follow-up/medication intake, hospitalizations and transfusions for SCD complications. Previously, Rohan et al showed that compliance in pediatric patients with chronic diseases such as cancer could be as low as 40.8% [49], and could be the result of many objective (e.g., age) [50], as well as socioeconomic factors [51]. In this study, we attempted to monitor compliance of the subjects by evaluating the plasma level of AZT at the POST intervals. Data were analyzed after completion of the study to maintain double-blind status. However, due to a combination of several factors such as the pharmacokinetics of AZT (which predominantly accumulates in the tissues, where only a very small amount (about 1%) leaks into the serum) [52, 53], its short (11 - 14 h) plasma half-life, and time between last administration of the drug and the patients’ visits when samples were collected, it appears that such an approach was not sufficient to adequately monitor compliance of the subjects.
Conclusions
As we acknowledged above, underlying cellular and molecular mechanisms of our observations should be studied further in details. However, data suggest that anti-inflammatory outcomes associated with use of AZT may have clinical importance in the treatment, and perhaps prevention, of the conditions that may potentially lead to inflammatory “vicious cycles”, and that AZT could be considered for, at least, the co-treatment of many pathological conditions where other anti-inflammatory agents may interfere with adequate host response.
| Supplementary Material | ▴Top |
Suppl 1. Demographic data of the subjects.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by the Navicent Health Foundation and Mercer University Seed Grant.
Conflict of Interest
Authors declare no conflict of interest.
Informed Consent
Informed consents were obtained.
Author Contributions
PNU and VSS: conception and design of the work; sample and data acquisition, data analysis and interpretation. FCD: data analysis and interpretation. ONU: data acquisition, analysis, and interpretation. JNS, WTU and OVS: data acquisition and analysis. All authors participated in the drafting, revising and final approval of the manuscripts.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
APC: allophycocyanin; CD: cluster of differentiation; CRP: C-reactive protein; CXCL8: CXC motif chemokine ligand 8; Cy5.5: cyanine 5.5; Cy7: cyanine 7; Cys: cystatin C; DAMP: damage-associated molecular patterns; FITC: fluorescein isothiocyanate; FS/SS: forward scatter/side scatter; ICAM: intercellular adhesion molecule; IGFBP: insulin-like growth factor-binding protein; IL1β: interleukin 1 beta; IL6: interleukin 6; LDH: lactate dehydrogenase; MMP: matrix metalloproteinases; MPO: myeloperoxidase; MRP8/14: myeloid-related protein 8/14; Myo: myoglobin; NGAL: lipocalin A; OPN: osteopontin; PE: phycoerythrin; PerCP: peridinin chlorophyll protein; SAA: serum amyloid A; SCD: sickle cell disease; TNFα: tumor necrosis factor alpha; VCAM: vascular cell adhesion molecule
| References | ▴Top |
- Brugnara C, de Franceschi L, Alper SL. Inhibition of Ca(2+)-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J Clin Invest. 1993;92(1):520-526.
doi pubmed - Dembele AK, Lapoumeroulie C, Diaw M, Tessougue O, Offredo L, Diallo DA, Diop S, et al. Cell-derived microparticles and sickle cell disease chronic vasculopathy in sub-Saharan Africa: A multinational study. Br J Haematol. 2021;192(3):634-642.
doi pubmed - Gardner RV. Sickle Cell Disease: Advances in Treatment. Ochsner J. 2018;18(4):377-389.
doi pubmed - Kato GJ. Defective nitric oxide metabolism in sickle cell disease. Pediatr Blood Cancer. 2015;62(3):373-374.
doi pubmed - Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383-1389.
doi pubmed - Ren H, Ghebremeskel K, Okpala I, Lee A, Ibegbulam O, Crawford M. Patients with sickle cell disease have reduced blood antioxidant protection. Int J Vitam Nutr Res. 2008;78(3):139-147.
doi pubmed - Romana M, Connes P, Key NS. Microparticles in sickle cell disease. Clin Hemorheol Microcirc. 2018;68(2-3):319-329.
doi pubmed - Antonioli E, Guglielmelli P, Pieri L, Finazzi M, Rumi E, Martinelli V, Vianelli N, et al. Hydroxyurea-related toxicity in 3,411 patients with Ph'-negative MPN. Am J Hematol. 2012;87(5):552-554.
doi pubmed - Chaine B, Neonato MG, Girot R, Aractingi S. Cutaneous adverse reactions to hydroxyurea in patients with sickle cell disease. Arch Dermatol. 2001;137(4):467-470.
- Stegelmann F, Wille K, Busen H, Fuchs C, Schauer S, Sadjadian P, Becker T, et al. Significant association of cutaneous adverse events with hydroxyurea: results from a prospective non-interventional study in BCR-ABL1-negative myeloproliferative neoplasms (MPN) - on behalf of the German Study Group-MPN. Leukemia. 2021;35(2):628-631.
doi pubmed - Friend A, Girzadas D. Acute Chest Syndrome. In: StatPearls. Treasure Island (FL), 2021.
- Zhang L, Su Z, Zhang Z, Lin J, Li DQ, Pflugfelder SC. Effects of azithromycin on gene expression profiles of proinflammatory and anti-inflammatory mediators in the eyelid margin and conjunctiva of patients with meibomian gland disease. JAMA Ophthalmol. 2015;133(10):1117-1123.
doi pubmed - Beigelman A, Mikols CL, Gunsten SP, Cannon CL, Brody SL, Walter MJ. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir Res. 2010;11:90.
doi pubmed - Cymbala AA, Edmonds LC, Bauer MA, Jederlinic PJ, May JJ, Victory JM, Amsden GW. The disease-modifying effects of twice-weekly oral azithromycin in patients with bronchiectasis. Treat Respir Med. 2005;4(2):117-122.
doi pubmed - Tokgoz B, Sari HI, Yildiz O, Aslan S, Sipahioglu M, Okten T, Oymak O, et al. Effects of azithromycin on cyclosporine-induced gingival hyperplasia in renal transplant patients. Transplant Proc. 2004;36(9):2699-2702.
doi pubmed - Gabriel AS, Ahnve S, Gnarpe H, Gnarpe J, Martinsson A. Azithromycin therapy in patients with chronic Chlamydia pneumoniae infection and coronary heart disease: immediate and long-term effects on inflammation, coagulation, and lipid status in a double-blind, placebo-controlled study. Eur J Intern Med. 2003;14(8):470-478.
doi pubmed - Schogler A, Kopf BS, Edwards MR, Johnston SL, Casaulta C, Kieninger E, Jung A, et al. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur Respir J. 2015;45(2):428-439.
doi pubmed - Gielen V, Johnston SL, Edwards MR. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010;36(3):646-654.
doi pubmed - Jiang K, Chen H, Fang Y, Chen L, Zhong C, Bu T, Dai S, et al. Exosomal ANGPTL1 attenuates colorectal cancer liver metastasis by regulating Kupffer cell secretion pattern and impeding MMP9 induced vascular leakiness. J Exp Clin Cancer Res. 2021;40(1):21.
doi pubmed - Ghosh A, Pechota A, Coleman D, Upchurch GR, Jr., Eliason JL. Cigarette smoke-induced MMP2 and MMP9 secretion from aortic vascular smooth cells is mediated via the Jak/Stat pathway. Hum Pathol. 2015;46(2):284-294.
doi pubmed - Luo J, Qiao F, Yin X. Hypoxia induces FGF2 production by vascular endothelial cells and alters MMP9 and TIMP1 expression in extravillous trophoblasts and their invasiveness in a cocultured model. J Reprod Dev. 2011;57(1):84-91.
doi pubmed - Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63(17):5224-5229.
- Cione E, Piegari E, Gallelli G, Caroleo MC, Lamirata E, Curcio F, Colosimo F, et al. Expression of MMP-2, MMP-9, and NGAL in tissue and serum of patients with vascular aneurysms and their modulation by statin treatment: A Pilot Study. Biomolecules. 2020;10(3):359.
doi pubmed - Cai X, Ahmad G, Hossain F, Liu Y, Wang X, Dennis J, Freedman B, et al. High-Density lipoprotein (HDL) inhibits serum amyloid A (SAA)-induced vascular and renal dysfunctions in apolipoprotein E-deficient mice. Int J Mol Sci. 2020;21(4):1316.
doi pubmed - Price WA, Moats-Staats BM, Stiles AD. Pro- and anti-inflammatory cytokines regulate insulin-like growth factor binding protein production by fetal rat lung fibroblasts. Am J Respir Cell Mol Biol. 2002;26(3):283-289.
doi pubmed - Hjortebjerg R, Thomsen KL, Agnholt J, Frystyk J. The IGF system in patients with inflammatory bowel disease treated with prednisolone or infliximab: potential role of the stanniocalcin-2 / PAPP-A / IGFBP-4 axis. BMC Gastroenterol. 2019;19(1):83.
doi pubmed - Wang JH, Su F, Wang S, Lu XC, Zhang SH, Chen D, Chen NN, et al. CXCR6 deficiency attenuates pressure overload-induced monocytes migration and cardiac fibrosis through downregulating TNF-alpha-dependent MMP9 pathway. Int J Clin Exp Pathol. 2014;7(10):6514-6523.
- Weiler J, Mohr M, Zanker KS, Dittmar T. Matrix metalloproteinase-9 (MMP9) is involved in the TNF-alpha-induced fusion of human M13SV1-Cre breast epithelial cells and human MDA-MB-435-pFDR1 cancer cells. Cell Commun Signal. 2018;16(1):14.
doi pubmed - Wilson WR, Anderton M, Choke EC, Dawson J, Loftus IM, Thompson MM. Elevated plasma MMP1 and MMP9 are associated with abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg. 2008;35(5):580-584.
doi pubmed - Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, Foell D, et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood. 2004;104(13):4260-4268.
doi pubmed - Viemann D, Barczyk K, Vogl T, Fischer U, Sunderkotter C, Schulze-Osthoff K, Roth J. MRP8/MRP14 impairs endothelial integrity and induces a caspase-dependent and -independent cell death program. Blood. 2007;109(6):2453-2460.
doi pubmed - Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, Klenner L, et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med. 2010;16(6):713-717.
doi pubmed - Schmidt M, Raghavan B, Muller V, Vogl T, Fejer G, Tchaptchet S, Keck S, et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol. 2010;11(9):814-819.
doi pubmed - Choi IY, Gerlag DM, Herenius MJ, Thurlings RM, Wijbrandts CA, Foell D, Vogl T, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74(3):499-505.
doi pubmed - Fassl SK, Austermann J, Papantonopoulou O, Riemenschneider M, Xue J, Bertheloot D, Freise N, et al. Transcriptome assessment reveals a dominant role for TLR4 in the activation of human monocytes by the alarmin MRP8. J Immunol. 2015;194(2):575-583.
doi pubmed - Morikis VA, Chase S, Wun T, Chaikof EL, Magnani JL, Simon SI. Selectin catch-bonds mechanotransduce integrin activation and neutrophil arrest on inflamed endothelium under shear flow. Blood. 2017;130(19):2101-2110.
doi pubmed - Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86(3):557-566.
doi pubmed - Holzinger D, Fassl SK, de Jager W, Lohse P, Rohrig UF, Gattorno M, Omenetti A, et al. Single amino acid charge switch defines clinically distinct proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1)-associated inflammatory diseases. J Allergy Clin Immunol. 2015;136(5):1337-1345.
doi pubmed - Stuart J, Stone PC, Akinola NO, Gallimore JR, Pepys MB. Monitoring the acute phase response to vaso-occlusive crisis in sickle cell disease. J Clin Pathol. 1994;47(2):166-169.
doi pubmed - Tumblin A, Tailor A, Hoehn GT, Mack AK, Mendelsohn L, Freeman L, Xu X, et al. Apolipoprotein A-I and serum amyloid A plasma levels are biomarkers of acute painful episodes in patients with sickle cell disease. Haematologica. 2010;95(9):1467-1472.
doi pubmed - Yuditskaya S, Tumblin A, Hoehn GT, Wang G, Drake SK, Xu X, Ying S, et al. Proteomic identification of altered apolipoprotein patterns in pulmonary hypertension and vasculopathy of sickle cell disease. Blood. 2009;113(5):1122-1128.
doi pubmed - Lannergard A, Larsson A, Kragsbjerg P, Friman G. Correlations between serum amyloid A protein and C-reactive protein in infectious diseases. Scand J Clin Lab Invest. 2003;63(4):267-272.
doi pubmed - Yip TT, Chan JW, Cho WC, Yip TT, Wang Z, Kwan TL, Law SC, et al. Protein chip array profiling analysis in patients with severe acute respiratory syndrome identified serum amyloid a protein as a biomarker potentially useful in monitoring the extent of pneumonia. Clin Chem. 2005;51(1):47-55.
doi pubmed - Ito T. PAMPs and DAMPs as triggers for DIC. J Intensive Care. 2014;2(1):67.
doi pubmed - Banerjee C, Yowtak J, Fridlyand D, Alleyne C, Jr. Acute spontaneous intracranial epidural haematoma and disseminated intravascular coagulation in a paediatric sickle cell patient. BMJ Case Rep. 2018;2018:bcr-2018-224504.
doi pubmed - Corvelli AI, Binder RA, Kales A. Disseminated intravascular coagulation in sickle cell crisis. South Med J. 1979;72(4):505-506.
doi pubmed - Iqbal MH, Abe O, Popilevsky F, Garewal V, Gillette P, Jamaleddine G, Zein JG. Hematologic parameters associated with worse outcome in critically ill sickle cell disease patients. Chest. 2008;134(4):61003.
doi - Bakanay SM, Dainer E, Clair B, Adekile A, Daitch L, Wells L, Holley L, et al. Mortality in sickle cell patients on hydroxyurea therapy. Blood. 2005;105(2):545-547.
doi pubmed - Rohan JM, Fukuda T, Alderfer MA, Wetherington Donewar C, Ewing L, Katz ER, Muriel AC, et al. Measuring Medication Adherence in Pediatric Cancer: An Approach to Validation. J Pediatr Psychol. 2017;42(2):232-244.
doi pubmed - McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. J Pediatr Psychol. 2003;28(5):323-333.
doi pubmed - Snodgrass SR, Vedanarayanan VV, Parker CC, Parks BR. Pediatric patients with undetectable anticonvulsant blood levels: comparison with compliant patients. J Child Neurol. 2001;16(3):164-168.
doi pubmed - Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990;25(Suppl A):73-82.
doi pubmed - Lode H. The pharmacokinetics of azithromycin and their clinical significance. Eur J Clin Microbiol Infect Dis. 1991;10(10):807-812.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


