| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Short Communication
Volume 10, Number 4, August 2021, pages 196-201
Platelet Aggregation Assays Do Not Reliably Diagnose Platelet Delta Granule Storage Pool Deficiency
William T. Gunning IIIa, d, Lorene Yoxtheimera, b, Mary R. Smitha, c
aDepartment of Pathology, University of Toledo, Toledo, OH, USA
bCurrent Address: Department of Pathology and Laboratory Medicine, Tulane University, New Orleans, LA, USA
cDepartment of Medicine, University of Toledo, Toledo, OH, USA
dCorresponding Author: William T. Gunning III, Department of Pathology, College of Medicine and Life Sciences, 3000 Arlington Avenue, MS 1090 Toledo, OH 43614, USA
Manuscript submitted March 30, 2021, accepted June 15, 2021, published online July 28, 2021
Short title: Platelet Aggregation and Platelet δ-SPD
doi: https://doi.org/10.14740/jh832
| Abstract | ▴Top |
Background: Patients with platelet dysfunction disorders present with a variety of mucocutaneous bleeding symptoms including easy bruising, frequent epistaxis, bleeding gums upon tooth brushing and for women, heavy menstrual bleeding. Available laboratory assays to evaluate platelet function include the platelet function analyzer (PFA) and in larger centers with coagulation laboratories, light transmission platelet aggregometry (LTA) analyses. Both assays are known to have a number of limitations, especially in the diagnosis of platelet delta granule storage pool deficiency (δ-SPD). δ-SPD is an underdiagnosed condition caused by decreased numbers of platelet dense granules (DGs) and is best diagnosed by electron microscopy (EM). Patients with platelet δ-SPD have a decreased response to low levels of the agonist adenosine diphosphate (ADP) in the second wave of light transmittance with LTA or decreased ADP secretion by fluorescence lumiaggregometry. There are few reports that have evaluated patients with δ-SPD and their respective LTA results. One report published in 1987 described normal LTA assays in 23% of patients with δ-SPD; a more recent report described LTA as having the sensitivity to detect only about 52% of patients with δ-SPD. The purpose of our study was intended to review the LTA and EM results of patients suspected of having a platelet function disorder at our institution for comparison with previously published studies.
Methods: Our study included 344 patients who had been evaluated by both LTA and whole mount EM. Aggregometry utilized five agonists: ADP, epinephrine, collagen, arachidonic acid, and ristocetin. DGs were enumerated in 100 whole-mounted platelets to determine a mean number of dense granules per platelet (DGs/PL).
Results: Seventy-seven percent of our patients were found to have δ-SPD (264/344); 68% (179/264) of these subjects had an abnormal platelet LTA. Thirty-two percent (85/264) of our patients had normal LTA results but were found to have δ-SPD with a mean of 2.54 ± 0.15 DG/PL (normal = 4 - 6 DG/PL).
Conclusion: These data confirm previous reports suggesting the utilization of LTA alone in patients with histories of unexplained bleeding may miss the diagnosis of platelet δ-SPD. It is, therefore, prudent to assess platelet DG number by EM, especially if platelet LTA assessment is normal.
Keywords: Platelet dysfunction; Storage pool deficiency; Light transmission aggregometry; Electron microscopy
| Introduction | ▴Top |
Mucocutaneous bleeding is indicative of a potential platelet dysfunction disorder, and identifying the etiology of this bleeding is often challenging [1-3]. Mucocutaneous bleeding manifests as easy bruising, epistaxis, and for women, heavy menstrual bleeding, which are common in the general population [4]. Platelet dysfunction disorders include a number of well-recognized genetic conditions with von Willebrand Disease (vWD) regarded as the most common. It has a reported incidence of 1% of the population and can have a number of genetic variations with more than 300 single nucleotide polymorphisms [5]. Other inherited platelet dysfunction disorders can be caused by defects in platelet receptors or defects in signal transduction such as Bernard-Soulier and Glanzmann’s thrombasthenia. A significant body of literature has established a deficiency of platelet dense granules (DGs) in Hermansky-Pudlak, Chediak-Higashi, Wiskott-Aldrich, thrombocytopenia and absence of radius (TAR), and Jacobsen/Paris-Trousseau syndromes as the etiology of mucocutaneous bleeding. A deficiency of platelet DGs is commonly referred to as dense (delta) granule storage pool deficiency (δ-SPD). The prevalence of platelet dysfunction disorders is unknown and many functional disorders may go undetected, especially if the disorder is mild [2, 4].
One of the essential laboratory tools employed to diagnose platelet dysfunction is a series of aggregation assays that utilize agonists known to activate platelets. The gold standard for aggregation assays has been light transmission aggregometry (LTA) with challenge to aliquots of platelet rich plasma by the agonists: adenosine diphosphate (ADP), epinephrine, collagen, arachidonic acid, and ristocetin [6-8].
The gold standard of diagnosing δ-SPD has been the use of air-dried, whole-mounted platelets for enumeration of DGs per platelet (DGs/PL) by electron microscopy (EM) [9, 10]. Algorithms used in the assessment of diagnosing platelet dysfunction generally indicate use of platelet LTA prior to investigation using EM [11-13]. Suspicion for δ-SPD by LTA is usually triggered by abnormal responses to the agonists ADP and/or epinephrine [14, 15]. Unfortunately, platelet aggregation assays are not sensitive for detection of δ-SPD [11, 14-16]. Reports by Nieuwenhuis et al (1987) and Israels et al (1990) established the inability to detect many cases δ-SPD by LTA [14, 15]. More recently, Woods et al have described a study in which more than 50% of pediatric and juvenile patients with δ-SPD demonstrated normal LTAs [16]. In 2018, Brunet et al reported LTA and lumiaggregometry had only about 52% and 70% sensitivity for δ-SPD, respectively [11].
There are numerous reports in the literature regarding platelet function assays such as LTA and other methodologies for evaluation of the patient with unexplained bleeding, but only a handful of studies that have specifically compared LTA results with EM determination of DGs/PL content as cited above. All previous investigations have had a limited number of subjects with a significant number of normal LTA results for patients with documented δ-SPD. The purpose of this retrospective study was intended to evaluate substantially more patients than any previous study and confirm previous study results that for at least LTA platelet function tests, a significant number of patients with platelet DG deficiency will not be diagnosed correctly without the use of whole-mounted platelet EM. To our knowledge, this is the largest study of its kind to assess the hypothesis that LTA is unreliable for diagnosis of δ-SPD.
| Materials and Methods | ▴Top |
Ethical issues
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected by prior approval from the Institutional Review Board of The University of Toledo Medical Center. All relevant patient information obtained from medical records was de-identified prior to assessment. All methodologies employed to analyze blood samples followed guidelines established by the College of American Pathologist (CAP, Chicago, IL, USA) and the Clinical Laboratory Improvement Amendments (CLIA, CDC, Atlanta, GA, USA).
Study design
This was a retrospective study comparing data generated during platelet function assessment of patients with unexplained bleeding at our institution for comparison to previously reported data suggesting that LTA may not be adequate for diagnosis of δ-SPD. All patients included in this study were suspected of having a platelet dysfunction disorder based upon clinical symptoms and laboratory screening tests. Specifically, we collected laboratory results for patients only if both platelet LTA studies and EM had been performed. Patients with LTA results suggestive of aspirin effect or with platelet counts below 100,000 were excluded. A total of 344 patients suspected of having a platelet dysfunction disorder had results for both assays over an 8-year period. Patients were categorized by the results of EM analysis as δ-SPD or within normal limits. Our laboratory has established normal range dense δ-granule values utilizing well-vetted control subjects who met the following criteria: screened by extensive bleeding questionnaires, normal complete blood cell counts, and normal platelet function analysis including platelet aggregation assays, and assessed for an extracted ATP/ADP ratio (< 2.0) via firefly luciferase assay [17, 18]. Our normal range of DG/PL is 4 - 6 (4.60 ± 0.31 DG/PL in our lab), consistent with values established in the literature [9, 11, 19]. Our lowest value for normal is 3.68 DG/PL, which is 3 standard errors of the mean for our control subjects. Thus, patients placed into the δ-SPD group had < 3.68 DG/PL, and those categorized as normal had a mean of 3.68 DG/PL or greater.
Platelet aggregation studies
Platelet aggregation studies required blood collection in 3.2% sodium citrate blue top tubes. Nine parts of whole blood were added to one part of anticoagulant in these tubes. The total blood draw was approximately 20 mL, divided into six vials and gently mixed.
Subsequently, blood vials were centrifuged at 900 rpm for 10 min at room temperature to obtain platelet rich plasma (PRP). The PRP pipetted from the six tubes was placed into a single tube and allowed to rest for 30 min; samples were centrifuged again at 3,500 rpm for 10 min at room temperature to concentrate the PRP and remove the platelet poor plasma (PPP). A cell counter was utilized to determine the PRP cell concentration and platelet number adjusted to 250,000 to 350,000 platelets/mL, using the PPP if the platelet count exceeded 350,000/mL.
A turbidimetric light transmittance aggregometer (Helena AggRAM, Beaumont, TX) was utilized in this study; neither a whole blood impedance aggregometer nor a lumiaggregometer was available for use. For each agonist assay, 450 µL of PRP was pipetted into cuvettes containing magnetic stir bars and allowed to rest for 1 min. Subsequently, 50 µL of the following reagents was added to each cuvette: ADP (20, 5, and 2.5 µL), epinephrine (5 and 10 µL), collagen (10 µg/mL), arachidonic acid (500 µg/mL), and ristocetin (1 and 0.5 mg/mL) [6, 20]. Each spectrophotometric assay totaled 300 s with the exception of epinephrine, which was measured for 600 s. Samples generating abnormal test results were retested to ensure the accuracy of the results as well as confirm that reagents and assays were functioning properly. Determining abnormal results in our lab included the subjective assessment of LTA instrument slope for each agonist with the percent aggregation (generally less than 70%). There is no standardized cut off values for responses to reagents; anything less than 70% maximal response was considered low except for our lowest concentration ADP curve, which may show only a primary wave in a normal subject. If there is disaggregation following initial primary wave response to lowest concentration ADP, we also considered this abnormal.
EM
Peripheral blood was collected in acid citrate dextrose Vacutainer® blood collection vials (yellow top ACD tubes, solution A or B, Becton Dickinson, Franklin Lakes, NJ). PRP was obtained by centrifugation at room temperature for 15 min at 200 g for preparation of platelet whole mounts [21, 22]. Twenty µL of PRP was placed upon parlodion coated copper EM grids and incubated for 10 min. Grids were then briefly washed with deionized water for 2 - 3 sec, blotted gently with filter paper, and air-dried. Preparations were assessed using a FEI Tecnai G2 Spirit BioTwin transmission electron microscope (Hillsboro, OR) at 80 kV. The mean number of DG/PL was determined by enumeration of the total number of DGs from 100 consecutive PLs observed. Platelets partially obscured by a grid bar or that exhibited preparation artifacts were excluded.
Statistics
Descriptive statistics were calculated using SigmaPlot 12.0 software (Systat Software, Inc., San Jose, CA, USA) to characterize subject demographics. Linear regression was used to determine potential relationships between platelet DGs or gender as dependent variables with the different platelet function agonists as continuous variables in our data. Both numerical and indexed data were utilized. The software was also used to produce graphs for the manuscript.
| Results | ▴Top |
Demographics
Of the 344 patients we identified for comparison of platelet aggregation LTA and EM test results, 280 were females with an average age of 36.5 ± 19.5, and 64 were males with an average age of 32.5 ± 26.2. δ-SPD was diagnosed in 264 (76.7%) patients: 218 females (average age = 37) and 46 males (average age = 33.5) (Table 1). Thirty-two percent (85/264) of the subjects with δ-SPD had normal LTA test results. Eighty patients with unexplained bleeding had a normal number of platelet DGs: 62 females (average age = 35) and 18 males (average age = 29) (Table 1). There were no significant differences between females and males for PL DG numbers.
 Click to view | Table 1. Patients With Delta Granule Storage Pool Deficiency and Normal Number of Dense Granules per Platelet |
Platelet aggregation studies
Platelet aggregation results were abnormal in 65.6% (143/218) of females with δ-SPD, whereas 78% of males diagnosed with δ-SPD demonstrated abnormal aggregation (Table 1). For patients with a normal number of DGs, 50% (21/62) of the women had an abnormal response to at least one agonist with LTA, while 61% of the men had an abnormal response (Table 1). There were significant differences between PL DG number and LTA agonist results. Figure 1 graphically demonstrates aggregation results for all patients evaluated for unexplained bleeding by both LTA and EM.
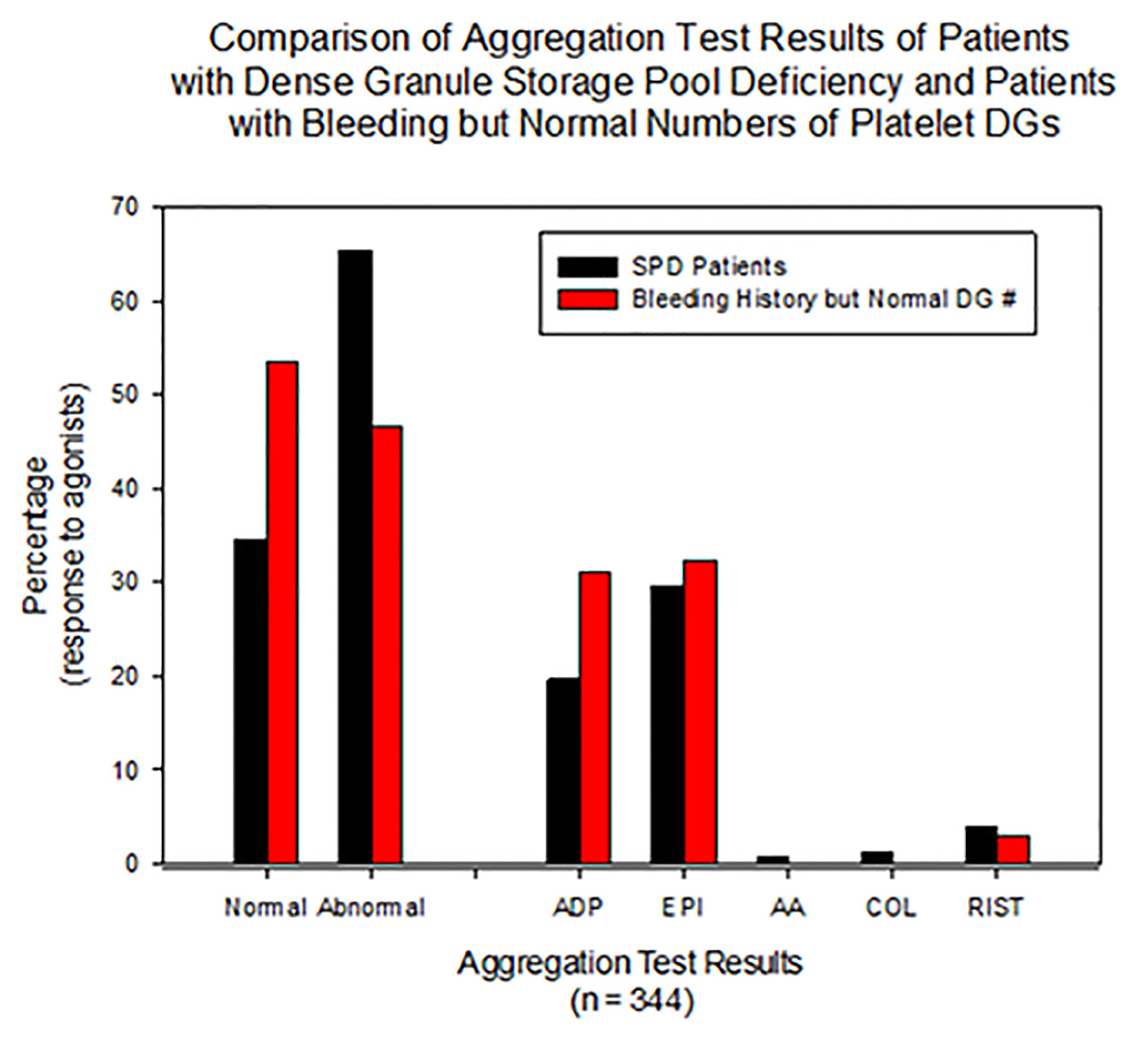 Click for large image | Figure 1. All patients presented with unexplained bleeding and were evaluated by both LTA and EM. This figure graphically demonstrates more than 30% of subjects with dense granule SPD have normal LTA results. For subjects with SPD, the agonist epinephrine was abnormal more often than for ADP as agonist. SPD: storage pool deficiency; LTA: luminescence transmission aggregometry; EM: electron microscopy. |
| Discussion | ▴Top |
The approach to diagnosing a suspected bleeding diathesis includes a detailed review of clinical bleeding symptoms and family history of bleeding, a bleeding assessment tool (BAT), determination of platelet count and size, and examination of a peripheral blood smear. Etiologies known to affect coagulation need to be ruled out (i.e., aspirin, uremia, autoimmune disorders, liver failure) [1, 23-25]. Standardized BATs, originally designed as a screening tool for vWD, are now utilized in the evaluation of the patient with unexplained bleeding [26, 27]. The BAT has been refined by ISTH working groups and is a valuable screening tool to determine the relative severity of abnormal bleeding, but it is unable to discriminate specific etiologies of unexplained bleeding [2]. The BAT has a poor correlation to δ-SPD [28]. Well-defined algorithms have been established to diagnose platelet dysfunction disorders [3, 6, 29, 30]. Even with the use of BATs and defined procedures to evaluate patients with bleeding diatheses, a laboratory diagnosis is not always straightforward, not well standardized, and may be prone to error [31-33].
Whole blood aggregometry (lumiaggregometry) to quantitate platelet secretion may have some advantages over LTA including requiring less blood and reduced technical demands [7]. However, this methodology has been reported to have significant variability and limited value in diagnosing platelet disorders [3, 8].
We did observe significant differences between PL DG number and LTA agonist responses but not for differences of gender. Although there were significantly more females in our study than males, it must be emphasized that this retrospective study was not a case-control study. We used data available to us where both LTA and EM had been used during diagnostic evaluation of patients with unexplained bleeding symptoms. There are reports in the literature that women have higher LTA aggregation responses to agonists than men, but this is likely related to higher numbers of circulating platelets for women, potentially related to hormone levels and platelet capacity to prevent excessive bleeding during menstruation [34, 35]. In our laboratory, we adjust the platelet count between 250,000 and 350,000/mL for standardization.
We did not observe significant differences for platelet DG number between women and men in this study. In our experience as a reference lab for evaluation of platelet δ-SPD, a general observation is that an equal number of prepubescent females and males have blood samples submitted for diagnosis. In the adult population of blood samples referred to us, approximately 75% are women. One could speculate why we see such a difference of gender but this report was intended to compare results obtained at our institution with previous reports in the literature to address the hypothesis that LTA is not reliable for diagnosis of platelet delta granule deficiency. We identified 32% of our patients being evaluated for unexplained bleeding had platelet δ-SPD and normal LTA response to all agonists tested. This is consistent with the reported literature and establishes the importance of utilization of EM during diagnostic workup of patients with unexplained bleeding symptoms.
Patients presenting with mucocutaneous bleeding symptoms such as easy bruising, epistaxis, gum bleeding with teeth brushing, significant post-surgical bleeding and for women, heavy menstrual bleeding are all suggestive of platelet dysfunction. Once assessed for quantitative issues (low platelets on complete blood cell count), screening tools including a BAT and PFA-100 may be utilized to qualify the severity of symptoms. Considering vWD is the most common platelet function disorder, the subsequent evaluation should include a von Willebrand profile assessment including determination of factor VIII, von Willebrand factor activity, and von Willebrand factor antigen. However, utilization of a platelet aggregation assay is essential to determine functional abnormalities. Whole blood aggregometry methods (lumiaggregometry or impedance aggregometry) have the advantages of requiring less sample and fewer steps but do not appear to be as reliable as LTA at this time. Diagnosing the patient with unexplained bleeding is unquestionably problematic as every laboratory test has exhibited limitations. This report demonstrates a significant number of patients with unexplained bleeding and normal platelet function assays may have δ-SPD. LTA remains the gold standard for assessment of unexplained bleeding related to functional abnormalities. It is essential that EM be employed in this workup, especially in patients with normal LTA results, to correctly diagnose conditions such as δ-SPD.
Conclusion
Evaluation of the patient with unexplained bleeding symptoms by LTA may result in false negative results for platelet dysfunction. Platelet whole mount EM assessment is an important tool to utilize in the diagnosis of such patients, especially if LTA results are normal for a patient presenting with significant clinical symptoms suggestive of a platelet function disorder.
Acknowledgments
None to declare.
Financial Disclosure
The authors have no financial disclosures as the project was unfunded.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
Not applicable.
Author Contributions
WTG: conception and design of the work, analysis of the data and writing the manuscript. LY: acquisition of the data, analysis and editing of the manuscript. MRS: acquisition of the data, analysis and editing of the manuscript. All authors shared equally in analysis, editing and accountability for all aspects of the work and all have granted final approval of the version to be published.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
BAT: bleeding assessment tool; CBC: complete blood cell count; DG: dense granule; LTA: luminescence transmission aggregometry; PFA: platelet function analyzer; PL: platelet; PPP: platelet poor plasma; PRP: platelet rich plasma; SPD: storage pool deficiency; vWD: von Willebrand disease
| References | ▴Top |
- Al-Huniti A, Kahr WH. Inherited platelet disorders: diagnosis and management. Transfus Med Rev. 2020;34(4):277-285.
doi pubmed - Mezzano D, Quiroga T. Diagnostic challenges of inherited mild bleeding disorders: a bait for poorly explored clinical and basic research. J Thromb Haemost. 2019;17(2):257-270.
doi pubmed - Hayward CPM, Moffat KA, Brunet J, Carlino SA, Plumhoff E, Meijer P, Zehnder JL. Update on diagnostic testing for platelet function disorders: What is practical and useful? Int J Lab Hematol. 2019;41(Suppl 1):26-32.
doi pubmed - Boender J, Kruip MJ, Leebeek FW. A diagnostic approach to mild bleeding disorders. J Thromb Haemost. 2016;14(8):1507-1516.
doi pubmed - Ng C, Motto DG, Di Paola J. Diagnostic approach to von Willebrand disease. Blood. 2015;125(13):2029-2037.
doi pubmed - Israels SJ. Laboratory testing for platelet function disorders. Int J Lab Hematol. 2015;37(Suppl 1):18-24.
doi pubmed - Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag. 2015;11:133-148.
doi pubmed - Le Blanc J, Mullier F, Vayne C, Lordkipanidze M. Advances in platelet function testing-light transmission aggregometry and beyond. J Clin Med. 2020;9(8):2636.
doi pubmed - White JG. Use of the electron microscope for diagnosis of platelet disorders. Semin Thromb Hemost. 1998;24(2):163-168.
doi pubmed - Dupuis A, Bordet JC, Eckly A, Gachet C. Platelet delta-Storage Pool Disease: An Update. J Clin Med. 2020;9(8):2508.
doi pubmed - Brunet JG, Iyer JK, Badin MS, Graf L, Moffat KA, Timleck M, Spitzer E, et al. Electron microscopy examination of platelet whole mount preparations to quantitate platelet dense granule numbers: Implications for diagnosing suspected platelet function disorders due to dense granule deficiency. Int J Lab Hematol. 2018;40(4):400-407.
doi pubmed - Selle F, James C, Tuffigo M, Pillois X, Viallard JF, Alessi MC, Fiore M. Clinical and laboratory findings in patients with delta-storage pool disease: a case series. Semin Thromb Hemost. 2017;43(1):48-58.
doi pubmed - Gresele P, Harrison P, Bury L, Falcinelli E, Gachet C, Hayward CP, Kenny D, et al. Diagnosis of suspected inherited platelet function disorders: results of a worldwide survey. J Thromb Haemost. 2014;12(9):1562-1569.
doi pubmed - Nieuwenhuis HK, Akkerman JW, Sixma JJ. Patients with a prolonged bleeding time and normal aggregation tests may have storage pool deficiency: studies on one hundred six patients. Blood. 1987;70(3):620-623.
doi pubmed - Israels SJ, McNicol A, Robertson C, Gerrard JM. Platelet storage pool deficiency: diagnosis in patients with prolonged bleeding times and normal platelet aggregation. Br J Haematol. 1990;75(1):118-121.
doi pubmed - Woods GM, Kudron EL, Davis K, Stanek J, Kerlin BA, O'Brien SH. Light Transmission Aggregometry Does Not Correlate With the Severity of delta-Granule Platelet Storage Pool Deficiency. J Pediatr Hematol Oncol. 2016;38(7):525-528.
doi pubmed - Holmsen H, Storm E, Day HJ. Determination of ATP and ADP in blood platelets: a modification of the firefly luciferase assay for plasma. Anal Biochem. 1972;46(2):489-501.
doi - Girotti S, Ferri E, Cascione ML, Orlandini A, Farina L, Nucci S, Di Graci F, et al. Methodological problems of direct bioluminescent ADP assay in platelets and erythrocytes. Anal Biochem. 1991;192(2):350-357.
doi - Gunning WT, 3rd, Raghavan M, Calomeni EP, Turner JN, Roysam B, Roysam S, Smith MR, et al. A morphometric analysis of platelet dense granules of patients with unexplained bleeding: a new entity of delta-microgranular storage pool deficiency. J Clin Med. 2020;9(6):1734.
doi pubmed - Hvas AM, Favaloro EJ. Platelet function analyzed by light transmission aggregometry. Methods Mol Biol. 2017;1646:321-331.
doi pubmed - White JG. The dense bodies of human platelets. Origin of serotonin storage particles from platelet granules. Am J Pathol. 1968;53(5):791-808.
- White JG. The dense bodies of human platelets: inherent electron opacity of the serotonin storage particles. Blood. 1969;33(4):598-606.
doi pubmed - Elbatarny M, Mollah S, Grabell J, Bae S, Deforest M, Tuttle A, Hopman W, et al. Normal range of bleeding scores for the ISTH-BAT: adult and pediatric data from the merging project. Haemophilia. 2014;20(6):831-835.
doi pubmed - Rydz N, James PD. The evolution and value of bleeding assessment tools. J Thromb Haemost. 2012;10(11):2223-2229.
doi pubmed - O'Brien SH. Bleeding scores: are they really useful? Hematology Am Soc Hematol Educ Program. 2012;2012:152-156.
doi pubmed - Tosetto A, Castaman G, Rodeghiero F. Assessing bleeding in von Willebrand disease with bleeding score. Blood Rev. 2007;21(2):89-97.
doi pubmed - Lowe GC, Lordkipanidze M, Watson SP, on behalf of the UK GAPP study group. Utility of the ISTH bleeding assessment tool in predicting platelet defects in participants with suspected inherited platelet function disorders. J Thromb Haemost. 2013;11(9):1663-1668.
doi pubmed - Nessle CN, Ghosal S, Mathews C, Taylor D, Myers J, Raj A, Panigrahi A. Weak correlation of bleeding scores to platelet electron microscopy: A retrospective chart review of pediatric patients with delta-storage pool disorder. Pediatr Blood Cancer. 2019;66(3):e27505.
doi pubmed - MacEachern K, Kaur H, Toukh M, Mumal I, Hamilton A, Scovil S, James P, et al. Comprehensive evaluation of hemostasis in normal women: impact on the diagnosis of mild bleeding disorders. Clin Appl Thromb Hemost. 2015;21(1):72-81.
doi pubmed - Sokkary NA, Venkateswaran L, Dietrich JE, Teruya J. Platelet function disorders and menorrhagia in adolescents: a review of laboratory diagnosis. J Pediatr Adolesc Gynecol. 2012;25(4):233-237.
doi pubmed - Bolton-Maggs PH, Favaloro EJ, Hillarp A, Jennings I, Kohler HP. Difficulties and pitfalls in the laboratory diagnosis of bleeding disorders. Haemophilia. 2012;18(Suppl 4):66-72.
doi pubmed - Favaloro EJ, Bonar R. Proficiency testing/external quality assurance for the PFA-100((R)). Clin Chem Lab Med. 2012;50(8):1393-1401.
doi pubmed - Flood VH. Perils, problems, and progress in laboratory diagnosis of von Willebrand disease. Semin Thromb Hemost. 2014;40(1):41-48.
doi pubmed - Ranucci M, Aloisio T, Di Dedda U, Menicanti L, de Vincentiis C, Baryshnikova E, Surgical, et al. Gender-based differences in platelet function and platelet reactivity to P2Y12 inhibitors. PLoS One. 2019;14(11):e0225771.
doi pubmed - Berlin G, Hammar M, Tapper L, Tynngard N. Effects of age, gender and menstrual cycle on platelet function assessed by impedance aggregometry. Platelets. 2019;30(4):473-479.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


