| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 10, Number 3, June 2021, pages 139-142
A Novel Case of Leflunomide-Induced Thrombotic Thrombocytopenic Purpura
Misty Dawn Shieldsa, b, d , William Paul Skelton IVa, b, Damian A. Labera, b, Michael Verboskya, Noman Ashrafb, c
aH. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA
bMorsani School of Medicine, University of South Florida, Tampa, FL, USA
cDepartment of Hematology Oncology, James A. Haley Veterans Hospital, Tampa, FL, USA
dCorresponding Author: Misty Dawn Shields, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, GME, Tampa, FL 33612, USA
Manuscript submitted April 3, 2021, accepted April 23, 2021, published online June 16, 2021
Short title: Leflunomide-Induced TTP
doi: https://doi.org/10.14740/jh837
| Abstract | ▴Top |
Leflunomide has not been previously associated with thrombotic thrombocytopenic purpura (TTP), a rare life-threatening clinical syndrome characterized by thrombotic microangiopathy (TMA) due to inability to cleave ADAMTS13. Here, we present the first case of leflunomide-induced TTP. Our patient developed encephalopathy, thrombocytopenia, anemia and hyperbilirubinemia 2 months after starting leflunomide. Schistocytes were noted on peripheral smear and ADAMTS13 activity was low (< 5%), consistent with acquired TTP. He received therapeutic plasma exchange, corticosteroids, rituximab and caplacizumab with normalization of hemolysis labs and ADAMTS13 activity. However, pancytopenia persisted, raising the suspicion for leflunomide toxicity. Oral cholestyramine treatment was empirically started before teriflunomide (a leflunomide metabolite) level was found to be elevated. Blood counts normalized after cholestyramine and have remained normal at last follow-up over a year later. This is the first reported case of TTP precipitated by leflunomide. Our case highlights the importance of recognizing drugs as an etiology of TMA and adds leflunomide to this list.
Keywords: Leflunomide; Thrombotic thrombocytopenic purpura; Drug-induced thrombotic microangiopathy; TMA
| Introduction | ▴Top |
Leflunomide is an immunomodulatory drug used for the treatment of autoimmune disorders such as rheumatoid arthritis (RA), psoriasis and multiple sclerosis. After absorption, leflunomide is converted into its active metabolite teriflunomide, which is a potent inhibitor of dihydroorotate dehydrogenase (DHODH) - a key enzyme involved in de novo pyrimidine synthesis. Leflunomide likely exerts its immunosuppressive effects by inhibiting T-lymphocyte proliferation through this pathway [1, 2].
Thrombotic thrombocytopenic purpura (TTP) is a rare life-threatening clinical syndrome characterized by thrombotic microangiopathy (TMA). Without urgent therapy, most patients die [3]. The underlying pathophysiology of TTP is severe depletion of the protease responsible for cleaving ultra-large von Willebrand factor (vWF) multimers known as ADAMTS13 (A Disintegrin and Metalloproteinase with Thrombospondin type 1 motif, member 13). Without ADAMTS13, ultra-large vWF multimers persist, resulting in binding and activation of circulating platelets. This in turn, leads to widespread intravascular microthrombi formation, consumptive thrombocytopenia, hemolytic anemia and multi-organ failure [3].
TTP can be either hereditary or more commonly, acquired. Circulating autoantibodies lead to depletion of ADAMTS13 in acquired TTP, whereas hereditary forms result from decreased production of ADAMTS13 [4]. Drugs are increasingly being recognized as an etiology of acquired TTP or drug-induced TMA (DITMA). Clinical manifestations of drug-induced TTP and DITMA overlap and the two terms are often used interchangeably; however, the underlying mechanisms differ. TTP is characterized by a severe ADAMTS13 deficiency which often responds to plasma exchange, whereas DITMA results from intravascular platelet-rich thrombi formation induced by immune or non-immune mechanisms after drug exposure. It does not usually respond to plasma exchange and discontinuation of the offending drug along with supportive care is required. Many drugs have been implicated in DITMA but evidence supporting a definite association of TMA is available for only a limited number of medications. Quinine is the most common cause of DITMA [5]. Others include chemotherapeutics (gemcitabine, oxaliplatin and mitomycin), antibiotics (vancomycin), narcotics (oxymorphone) and immunosuppressants (cyclosporine and tacrolimus) [5, 6].
To the best of our knowledge, we present the first case of leflunomide-induced TTP in a patient with RA.
| Case Report | ▴Top |
Investigations
A 78-year-old Caucasian man with hypertension and RA (on sulfasalazine and leflunomide) presented to another hospital with acute encephalopathy, lethargy and an erythematous rash. He was found to be profoundly thrombocytopenic and anemic.
Six months prior to admission, blood counts were normal (Table 1). Two months prior, he started taking leflunomide 20 mg daily for RA. Complete blood count at that time showed mild anemia with a normal platelet count (Table 1). He reduced leflunomide to 10 mg daily due to an episode of melena 2 weeks prior to presentation. On admission (day 1), he was disoriented, tachycardic, afebrile and without hypoxia. Initial laboratory tests showed platelet count 3,000/µL, hemoglobin 7.3 g/dL and total bilirubin 3.0 mg/dL (Table 1, Fig. 1).
 Click to view | Table 1. Pertinent Labs During the Patient’s Clinical Course |
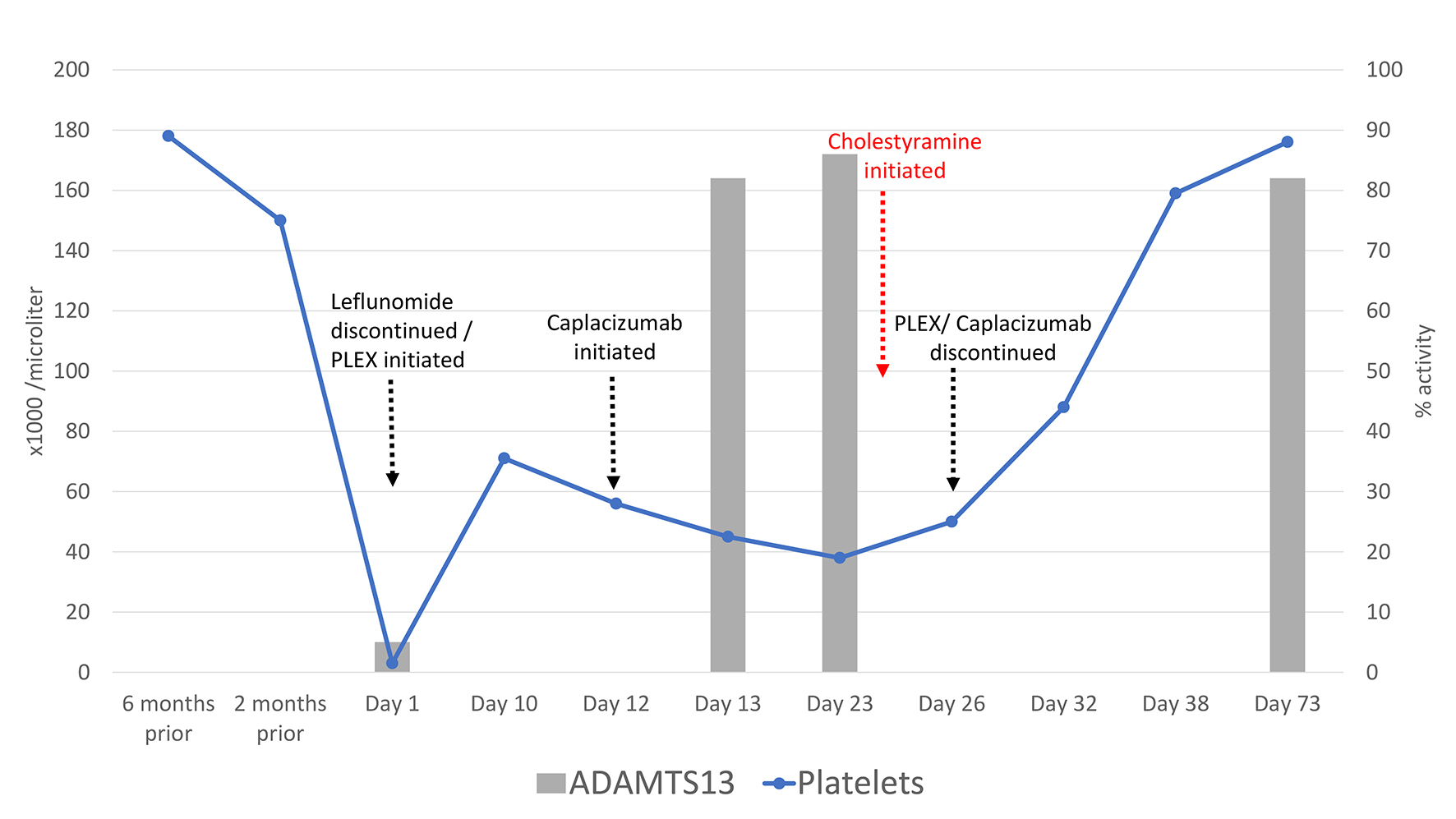 Click for large image | Figure 1. Pertinent labs and trends during the patient’s clinical course. |
Diagnosis
White blood cell (WBC) count, creatinine, vitamin B12, folate levels, partial thromboplastin and prothrombin times were normal. Peripheral blood smear revealed numerous schistocytes per high power field. Brain magnetic resonance imaging (MRI) was concerning for an acute left occipital stroke. Sulfasalazine, leflunomide and aspirin were held, and an ADAMTS13 activity level was obtained. Given suspicion of TTP, plasma exchange and intravenous methylprednisolone were started on day 1. Bone marrow biopsy performed on day 4 showed normal trilineage hematopoiesis, adequate iron stores and no blasts. The ADAMTS13 activity returned as < 5%, consistent with acquired TTP. However, no inhibitor/autoantibody was identified. Intravenous rituximab was initiated on day 9. His platelet count improved to 71,000/µL on day 10, but then declined to 56,000/µL, so he was initiated on caplacizumab, a vWF-directed antibody fragment, on day 12 (Table 1, Fig. 1).
On day 13, he was transferred to our tertiary care center. At admission to our hospital, his platelet count (45,000/µL) and hemoglobin (8.4 g/dL) remained low, but haptoglobin and lactate dehydrogenase (LDH) were normal (Table 1). WBC count, creatinine, reticulocyte index, immature platelet fraction, B12, folate, iron and fibrinogen were normal. Infectious serologies were negative for hepatitis C virus (HCV), hepatitis B virus (HBV) and human immunodeficiency virus (HIV). There was no clinical evidence of RA flare and rheumatologic workup was negative for other autoimmune disorders. Daily plasma exchange, caplacizumab and prednisone were continued. Repeat ADAMTS13 activity was 82% (day 13) and 86% (day 23). With normalization of LDH and ADAMTS13 activity, a steroid taper was initiated on day 23. Caplacizumab and plasma exchange were discontinued on day 26. Repeat bone marrow biopsy on day 27 demonstrated slightly hypercellular marrow with erythroid predominance, minimal dyspoiesis and no blasts. Flow cytometry and cytogenetics were normal. The patient remained pancytopenic with a normal LDH on day 23 (Table 1).
Treatment
Suspecting leflunomide toxicity, serum teriflunomide concentration was obtained and oral cholestyramine 8 g three times daily was empirically started on day 24. His blood counts began to improve 2 days later on day 26 (Table 1, Fig. 1). The teriflunomide level from day 23 returned elevated at 60 ng/mL [7].
| Follow-up and outcomes | ▴Top |
After 8 days of cholestyramine therapy, repeat teriflunomide level on day 32 was undetectable, and all blood counts were improving (Table 1, Fig. 1). He was discharged home with 11 additional days of cholestyramine. Follow-up on day 38 showed normalization of platelet and WBC counts (Table 1). To date, the patient has not relapsed with his last follow-up over a year from initial diagnosis.
| Discussion | ▴Top |
This is the first reported case of TTP precipitated by leflunomide. The diagnosis of TTP was established by the presence of thrombocytopenia, microangiopathic hemolytic anemia and a very low-level activity of ADAMTS13 (< 5%). Treatment with urgent plasma exchange, high-dose corticosteroids, rituximab and caplacizumab was highly effective for TTP with normalization of LDH and recovery of ADAMTS13 activity. However, platelet counts did not normalize. Delayed platelet count recovery with normal ADAMTS13 activity and lack of evidence of an active autoimmune flare or malignancy, raised our suspicion for leflunomide toxicity. Hence, cholestyramine was empirically administered to expedite clearance of the presumed toxic teriflunomide metabolite. The diagnosis was later confirmed by the elevated teriflunomide level while pancytopenic, and prompt improvement in hematologic parameters after clearance of the toxic metabolite. It is noteworthy that although leflunomide itself has a half-life of approximately 15 days, its active metabolite teriflunomide can persist for up to 2 years in the plasma due to extensive enterohepatic recirculation [8]. Elimination can be dramatically enhanced by preventing enterohepatic recirculation with the use of binders such as charcoal or cholestyramine, as demonstrated here in our case [8].
The pathophysiology of drug-mediated TTP generally involves either a toxic dose-related reaction, immune reaction, or metabolism-mediated mechanism [6]. Leflunomide can cause pancytopenia as we described in our case, by inhibiting DHODH. However, this does not explain our patient’s presentation with TTP. An immune reaction seems less likely given the absence of ADAMTS13 inhibitors, negative direct Coomb’s testing, normal complement levels and the lack of clinical evidence of an underlying rheumatologic flare at presentation. We posit toxic dose-related reaction of leflunomide as the most likely precipitant, supported by the elevated metabolite level on day 23, and normalization of the blood counts upon clearing teriflunomide with cholestyramine. Since ADAMTS13 is synthesized in the liver, it is possible that leflunomide-induced hepatotoxicity caused its severe deficiency, albeit the liver enzymes remained normal. Definite causal association of an adverse drug reaction can be established by drug re-challenge, but it would be unsafe and potentially life-threatening in TTP and should not be attempted. The above evidence supports a probable association of leflunomide with TTP.
The Oklahoma TTP-HUS Registry, a comprehensive database including over 1,300 reports of more than 300 drugs with their causal relation to drug-induced thrombocytopenia (TTP and TMA), was queried by direct correspondence [9, 10]. There were no preexisting reports of definite causal association of TMA/TTP with leflunomide. Previous reports have noted pancytopenia with macrocytic anemia and schistocytes, thought to be secondary to drug-induced megaloblastic anemia [11]. A case of TMA with moderately reduced ADAMTS13 (35%) has been reported, but it was in the setting of hepatic and renal toxicity with long-term use of a combination of leflunomide and methotrexate [12].
Learning points
Our case highlights the importance of recognizing drugs as an etiology of TMA. Once suspected, the offending agent must be promptly discontinued, and supportive care initiated. Drug-induced TTP and DITMA have overlapping clinical presentations, but distinct underlying mechanisms and differing response to treatment. Measurement of ADAMTS13 activity can differentiate the two; however, testing and results may not be readily available. Therapeutic plasma exchange should not be delayed if there is any suspicion of TTP. In patients with an initial platelet response to plasma exchange with subsequent worsening, or no response at all, alternative etiologies such as drug-induced toxicity should be considered. Further studies are needed to confirm the association between leflunomide and TTP.
In conclusion, this well-documented case of TTP associated with leflunomide adds to the medical knowledge. We hope it will increase awareness of rare drug complications.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
Study conception and design: Shields MD, Skelton WP, Laber DA, Ashraf N; data collection: Shields MD, Skelton WP; analysis and interpretation of results: Shields MD, Skelton WP, Laber DA, Verbosky M, Ashraf N; draft manuscript preparation: Shields MD, Skelton WP, Ashraf N. All authors reviewed the results and approved the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Davis JP, Cain GA, Pitts WJ, Magolda RL, Copeland RA. The immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase. Biochemistry. 1996;35(4):1270-1273.
doi pubmed - Aly L, Hemmer B, Korn T. From leflunomide to teriflunomide: drug development and immunosuppressive oral drugs in the treatment of multiple sclerosis. Curr Neuropharmacol. 2017;15(6):874-891.
doi pubmed - Sadler JE. Pathophysiology of thrombotic thrombocytopenic purpura. Blood. 2017;130(10):1181-1188.
doi pubmed - Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339(22):1578-1584.
doi pubmed - Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood. 2015;125(4):616-618.
doi pubmed - George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666.
doi pubmed - Rule GS, Rockwood AL, Johnson-Davis KL. Quantitation of teriflunomide in human serum/plasma across a 40,000-fold concentration range by LC/MS/MS. Methods Mol Biol. 2016;1383:195-203.
doi pubmed - Rozman B. Clinical pharmacokinetics of leflunomide. Clin Pharmacokinet. 2002;41(6):421-430.
doi pubmed - George JN, Aster RH. Drug-induced thrombocytopenia: pathogenesis, evaluation, and management. Hematology Am Soc Hematol Educ Program. 2009;2009:153-158.
doi pubmed - Reese JA, Bougie DW, Curtis BR, Terrell DR, Vesely SK, Aster RH, George JN. Drug-induced thrombotic microangiopathy: Experience of the Oklahoma Registry and the BloodCenter of Wisconsin. Am J Hematol. 2015;90(5):406-410.
doi pubmed - Toyokawa Y, Kingetsu I, Yasuda C, Yasuda J, Yoshida K, Kurosaka D, Yamada A. Pancytopenia, including macrocytic anemia, associated with leflunomide in a rheumatoid arthritis patient. Mod Rheumatol. 2007;17(5):436-440.
doi pubmed - Pliquett RU, Lubbert C, Schafer C, Girndt M. Thrombotic microangiopathy and liver toxicity due to a combination therapy of leflunomide and methotrexate: a case report. J Med Case Rep. 2020;14(1):26.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


