| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 12, Number 6, December 2023, pages 255-267
Haploidentical and Matched Sibling Transplantation for Acute Myeloid Leukemia: A Hospital-Based Study
Juan C. Baenaa, b, Maria C. Rosalesa, Mayra Estacioc, Alejandra Hidalgoc, Elizabeth Arrietab, d, e, Francisco J. Jaramillob, d, Eliana Manzic, Luis Gabriel Parra-Laraa, c , Joaquin D. Rosalesb, d
aFacultad de Ciencias de la Salud, Universidad Icesi, Cali, Colombia
bHematology Service, Department of Internal Medicine, Fundacion Valle del Lili, Cali, Colombia
cCentro de Investigaciones Clinicas (CIC), Fundacion Valle del Lili, Cali, Colombia
dBone Marrow Transplant Unit, Fundacion Valle del Lili, Cali, Colombia
eCorresponding Author: Elizabeth Arrieta, Hematology Service, Fundacion Valle del Lili, Cali 760032, Valle del Cauca, Colombia
Manuscript submitted July 7, 2023, accepted November 17, 2023, published online December 28, 2023
Short title: Transplantation for Acute Myeloid Leukemia
doi: https://doi.org/10.14740/jh1162
| Abstract | ▴Top |
Background: Allogeneic peripheral blood stem cell transplantation (PBSCT) has been increasing for the last years in Latin America. The objective of this study was to describe clinical outcomes in acute myeloid leukemia (AML) receiving allogeneic PBSCT between 2013 and 2019 in a single center of Cali, Colombia.
Methods: A retrospective cohort study was conducted in Fundacion Valle del Lili. Patients diagnosed with AML who received an allogeneic PBSCT between 2013 and 2019 using human leukocyte antigen (HLA)-matched sibling donors (MSDs) or haploidentical related donors (HRDs) with myeloablative conditioning regimen were included. Cases with diagnosis of promyelocytic leukemia, myelodysplastic syndrome-related AML and therapy-related AML were excluded. Data were obtained directly from the hospital PBSCT database and clinical records.
Results: A total of 50 patients were included (HRD, n = 32; MSD, n = 18). Sixty-two percent was in the first complete remission (CR1) at the time of the transplant, of which 26% were MSD and 74% were HRD. The European Group for Blood and Marrow Transplantation (EBMT) risk score was: 44% vs. 50% low, 28% vs. 28% intermediate and 28% vs. 22% high for MSD vs. HRD, respectively. Overall survival at 5 years for MSD was 62% (95% confidence interval (CI): 31-83%) and 43% (95% CI: 25-60%) for HRD. Event-free survival was 56% (95% CI: 26-78%) and 35.6% (95% CI: 18-53%), respectively. Non-relapse mortality at day-100 was 6% (95% CI: 0.8-35%) and 20% (95% CI: 9-39%). Relapse at5 years was 18% (95% CI: 4-58%) and 25% (95% CI: 10-52%). Overall mortality rate was 46%. The grade II-IV, III-IV acute graft-versus-host disease and severe chronic graft-versus-host disease was 44%, 11% and 12% for MSD, and 43%, 9% and 0% for HRD.
Conclusion: These results underline that MSD remains the first donor choice for AML patients in CR1 when available. HRDs are still our next option among alternative donors. It is necessary to find strategies that have a positive impact on those outcomes that markedly affect the quality of allogeneic PBSCT and the prognosis of patients. Comparative, randomized, prospective studies with longer follow-up of haploidentical allogeneic PBSCT with other donor types are required to definitely establish its role among alternative donors.
Keywords: Acute myeloid leukemia; Hematopoietic stem cell transplantation; Bone marrow transplantation; Survival; Colombia; Myeloablative conditioning regimen; Donors
| Introduction | ▴Top |
Acute myeloid leukemia (AML) is the most common form of acute leukemia among adults worldwide. The estimated new cases and deaths were 20,050 and 1,560 cases in 2022 for both sexes in the United States [1]. The overall 5-year survival rate in patients with AML was 28% during period 2010 to 2017 in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database [2].
Allogeneic hematopoietic stem cell transplantation (HSCT) is the most effective post-remission treatment for prevention of relapse in high and intermediate risk AML in first complete remission (CR1) [3]. Normally, human leukocyte antigen (HLA)-matched sibling donors (MSDs) are considered the optimal donors, but less than one-third of patients will have an available MSD [4]. For this reason, matched unrelated donors (MUDs) have been increasing their numbers in the last decade and registries worldwide now include more than about 40 million volunteer donors, most of them in North America and Europe [5]. However, the probability of finding a fully MUD varies on average between 16% and 75% depending on ethnicity [6, 7]. Also, lack of access limits this option in some regions [8].
The use of umbilical cord blood (UCB) grafts continues to decrease with the rise in numbers of haploidentical transplants performed and its high cost [7, 9]. When haploidentical related donors (HRDs) are used, improvements in conditioning regimens such as pre-transplant thymoglobulin and post-transplant cyclophosphamide (PT-Cy) have helped to decrease non-relapse mortality (NRM) and severe graft-versus-host disease (GvHD) incidence [10].
Allogeneic peripheral blood stem cell transplantation (PBSCT) has been increasing for the past years in Latin America. Nevertheless, transplant rate is five- to eight-fold lower than that in the North America and European regions [11]. Several Latin American centers have reported their results according to their resources [12]. The objective of this study was to describe clinical outcomes in patients with AML receiving allogeneic PBSCT between 2013 and 2019 using either MSD or PT-Cy-based haploidentical PBSCT in Cali, Colombia.
| Materials and Methods | ▴Top |
Design and setting
A retrospective, hospital-based, observational cohort study was conducted in the city of Cali, Colombia. Cali, capital of the Valle del Cauca Province, is the third largest city in the country with around 2,250,000 inhabitants in 2019 [13]. During a 5-year period 2013 - 2017, 346 new cases of myeloid leukemia were diagnosed in permanent residents of Cali with an age-standardized incidence rate of 3.1 for men and 2.1 for women per 100,000 person-years (world standard population) [14].
In the country, there are around 15 bone marrow transplant centers, two of them located in the city [15]. Unrelated HLA-matched or UCB transplantations are not performed in the country. In 2012, a total of 428 HSCTs were reported in the Colombian public health surveillance system, of which 61% (261) were autologous transplants, 32.2% (138) allogeneic transplants and 6.8% (29) transplants with an HLA-haploidentical donor [16].
Fundacion Valle del Lili is a high-complexity university hospital that serves as a reference center in southwestern Colombia. It is one of five hospitals that have integrated oncological services in the city. Supplementary Material 1 (www.thejh.org) shows the frequency of HSCT in the hospital.
A literature review was performed. Search terms were defined that described four key concepts: 1) AML; 2) HSCT; 3) MSD; 4) HRD; these terms were combined using Boolean operators. The search was conducted in MEDLINE (PubMed). Additional information was identified by manually screening the references of the retrieved literature reviews and of some of the articles retained.
Institutional Review Board approval
The Institutional Review Board - Comite de Etica en Investigacion Biomedica of the Fundacion Valle del Lili approved the study protocol (Protocol 974, Minutes 8-2016). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. Also, it was considered the regulations of Resolution 8430/1993 of the Ministry of Health of Colombia.
Patients and follow-up
Case definition
All patients aged ≥ 18 years with AML diagnosis who mostly were in CR1 at the time of their first allogeneic PBSCT using an MSD or HRD were included in this study.
Cases were obtained from the hospital HSCT database during the period from January 2013 to December 2019. The database includes information related to demographic, diagnosis, treatment and follow-up (vital status and last contact).
Exclusion criteria
Cases with higher than 10% missing data in clinical record and diagnosis of promyelocytic leukemia, myelodysplastic syndrome-related AML and therapy-related AML were excluded.
Transplantation procedures
HLA (A, -B, -C, -DR and - DQ loci) typing by PCR-SSOP-Luminex method was performed in donors and recipients. HLA disparity: A, B, C and DRB1, DQB1 between 5/10 and 10/10, negative panel reactive antibody (PRA) and donor-specific anti-HLA antibodies (DSA) tests, cytomegalovirus serostatus, donor-recipient ABO group and donor age and sex were considered in patients with several donors following institutional guidelines.
Hematopoietic stem cell harvest
Peripheral blood stem cell mobilization and collection protocol included granulocyte colony-stimulating factor (G-CSF) with filgrastim 10 µg/kg/day for 5 days after stem cells were cryopreserved using 5% dimethyl sulfoxide (DMSO) and stored at a temperature of -84 °C.
Conditioning regimens
Myeloablative conditioning (MAC) was applied in all patients with fludarabine (30 mg/m2 days -5 to -2), busulfan (110 mg/m2 days -5 to -2) plus 400 cGy fractionated total body irradiation (fTBI) (BUFLU TBI-400) or cyclophosphamide (Cy) (120 mg/kg) plus 1,320 cGy fTBI (Cy TBI-1320).
Supportive measures
Blood products and nutritional support were provided according to institutional guidelines. All blood products were filtered and radiated. A hemoglobin level < 6 g/dL and less than 10,000 platelets or any bleeding prompted transfusion. Parenteral nutrition was administered when applicable (i.e., grade III-IV mucositis).
GvHD prophylaxis
Institutional guideline included cyclosporine (CSP) and mycophenolate mofetil (MMF) or methotrexate (MTX), and post-transplant Cy (PT-Cy) 50 mg/kg/day on days +3 and +4 in HRD. CSP and MMF or MTX were used for MSD. G-CSF was applied from day +7 to neutrophil engraftment.
Antimicrobial prophylaxis
Acyclovir 400 mg every 12 h PO, and a single tablet of 160 mg of sulfamethoxazole and 800 mg of trimethoprim three times per week PO, posaconazole 300 mg every 12 h on day 1, followed by 300 mg daily PO were all administered starting the conditioning regimen. Patients did not receive antibiotic prophylaxis according to hospital protocol (cefepime 2,000 mg every 8 h in case of febrile neutropenia).
Follow-up
Vital status and the date of death or the last day of follow-up were determined using the HSCT database, general hospital mortality, hospital discharge, or the health system affiliation database (BDUA).
Clinical outcomes
The primary outcome of the study was overall survival (OS). Secondary outcomes included event-free survival (EFS), relapse-free survival (RFS), relapse, NRM, transplant-related mortality (TRM), acute and chronic GvHD [17]. All outcomes were measured since the date of HSCT.
EFS was defined as survival with no evidence of relapse or death. Relapse was defined as the reappearance of > 5% blasts on morphological evaluation in the bone marrow or an extramedullary site. NRM was defined as death without evidence of relapse or progression. TRM was defined as death due to any transplantation-related cause other than disease relapse. Acute and chronic GvHDs were defined according to Glucksberg standard criteria [18]. Neutrophil engraftment was defined as achieving an absolute neutrophil count > 0.5 × 109/L for three consecutive days, and platelet engraftment was defined as a platelet count > 20 × 109/L for 7 days unsupported by transfusion. Primary graft failure was defined as no evidence of engraftment or hematological recovery of donor cells, within the first month after transplant, without evidence of disease relapse.
The European Group for Blood and Marrow Transplantation (EBMT) risk score was calculated. It is based on patient and transplantation characteristics, which was developed in chronic myeloid leukemia patients and subsequently validated in other patient groups including AML [19].
Statistical analysis
A descriptive analysis of the sociodemographic and clinical variables was performed using measures of central tendency and dispersion. Quantitative variables were compared using the Mann-Whitney U test. Qualitative variables are shown as frequencies and percentages and were compared using the χ2 test or Fisher’s exact test.
Survival analysis was performed using the Kaplan-Meier method. OS was calculated using the date of allogeneic PBSCT and the date of death from any cause or the last day of follow-up (the last day of hospital care and the date of last contact recorded; the most recent date were used).
Relapse and NRM were summarized using cumulative incidence estimates. OS and EFS were compared according to the EBMT risk score using log-rank test.
A value of P < 0.05 was considered statistically significant. All analyses were performed using STATA® (Version 14.0, StataCorp LP, College Station, TX).
| Results | ▴Top |
A total of 50 patients (HRD, n = 32; MSD, n = 18) were included.
Patient characteristics
Patient, disease, and transplant characteristics are shown in Tables 1 and 2. Sixty-two percent were in CR1 (MSD: 26%, and HRD: 74%). Median age of recipient and donor was 38 years (interquartile range (IQR): 24 - 47) and 31 years (IQR: 22 - 41), respectively. Low, intermediate and high EBMT risk scores were 44%, 28% and 28% for MSD, and 50%, 28% and 22% for HRD.
 Click to view | Table 1. Patients and Hematopoietic Stem Cell Transplantation Characteristics (n = 50) |
 Click to view | Table 2. Mortality-Related Transplant Factors (n = 50) |
Transplant characteristics
MAC was performed mostly with BUFLU TBI-400 (63%), whereas 37% patients received Cy TBI-1320 (Table 2). Stem cell source was peripheral blood in all cases. Forty-seven patients had both neutrophil and platelet engraftment. The median neutrophil and platelet engraftment time was 17 days (IQR: 13 - 19) and 18 days (IQR: 14 - 24), respectively.
Clinical outcomes
Five-year OS for MSD was 62% (95% confidence interval (CI): 31-83%), and 43% (95% CI: 25-60%) for HRD (Fig. 1). There is no statistically significant difference between group (P = 0.142). OS in MSD using BUFLU TBI-400 vs. Cy TBI-1320 was 100% vs. 33% at 12 months, and 48% vs. 45% at 12 months for HRD. Supplementary Material 2 (www.thejh.org) shows the OS by conditioning regimens.
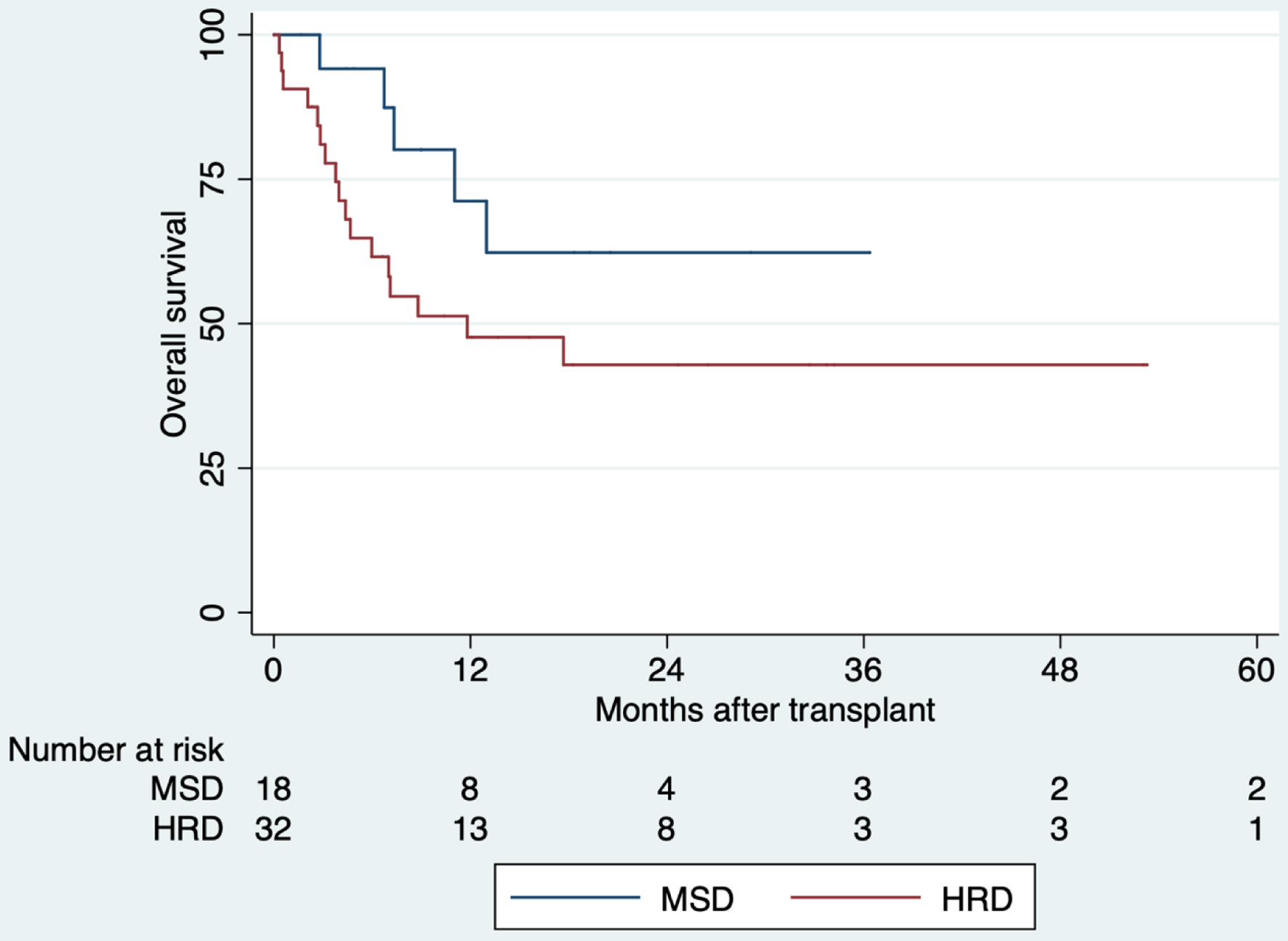 Click for large image | Figure 1. Comparison of overall survival of allogeneic hematopoietic stem cell transplantation, 2013 - 2019. There is no statistically significant difference between groups (P = 0.142). HRD: haploidentical related donor; MSD: HLA-matched sibling donor. |
EFS was 56% (95% CI: 26-78%) and 35.6% (95% CI: 18-53%), respectively. There is no statistically significant difference between group (P = 0.107). Five-year OS for MSD with EBMT risk score < 3 was 76% (95% CI: 30-91%) and 44% (95% CI: 7-78%) with EBMT risk score > 4, although there is no statistically significant difference (P = 0.619). In those with HRD, OS with EBMT score < 3 was 48% (95% CI: 27-66%), and 40% (95% CI: 5-64%) with EBMT risk score > 4 at 5 years of follow-up, also there is no statistically significant difference (P = 0.917). RFS was 77% (95% CI: 43-92%) for MSD and 57% (95% CI: 36-73%) for HRD (Fig. 2). There is no statistically significant difference between group (P = 0.188).
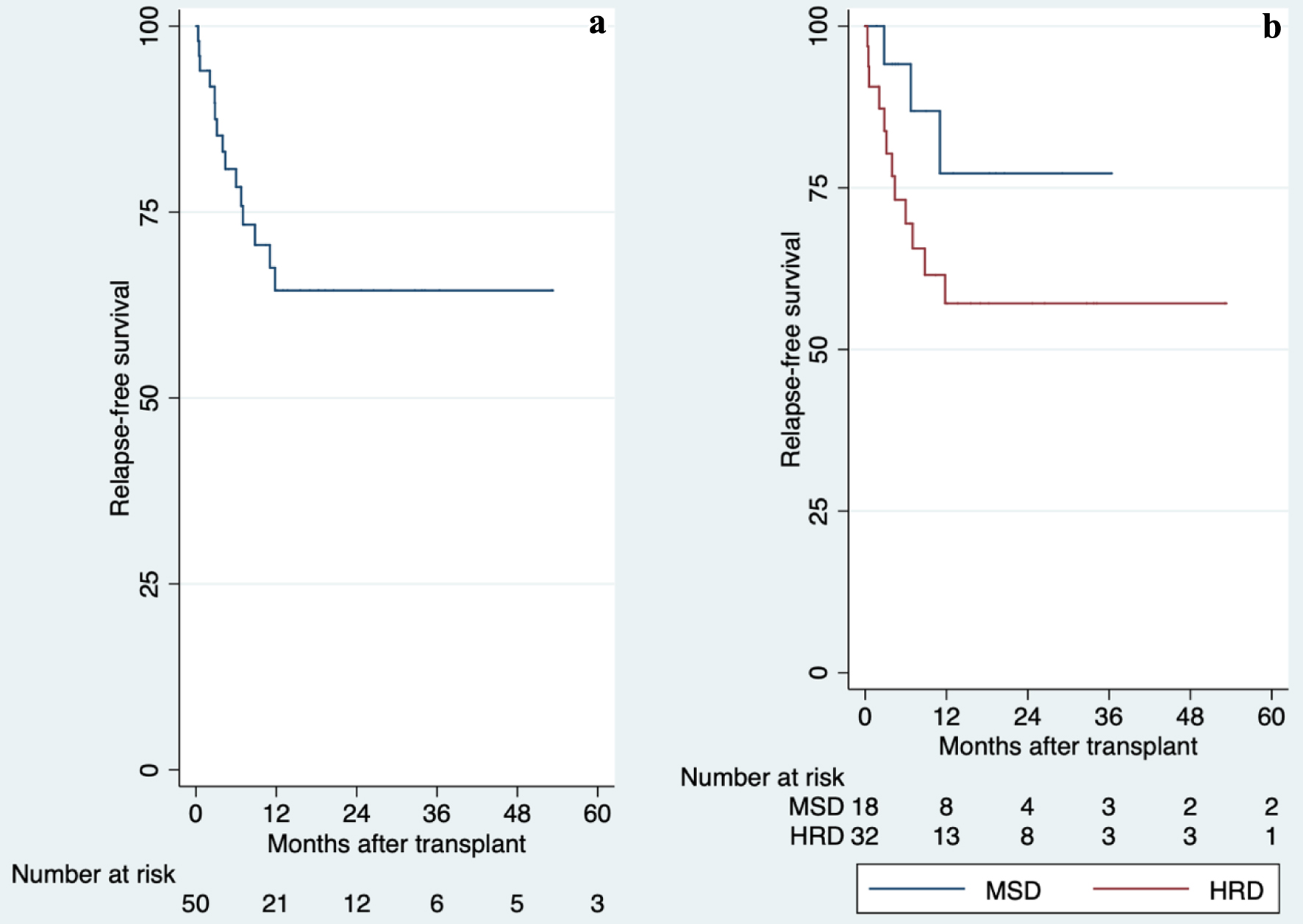 Click for large image | Figure 2. Overall and comparison of relapse-free survival in allogeneic hematopoietic stem cell transplantation, 2013 - 2019. (a) Overall of relapse-free survival. (b) Comparison of relapse-free survival. There is no statistically significant difference between groups (P = 0.188). HRD: haploidentical related donor; MSD: HLA-matched sibling donor. |
The day-100 NRM for MSD was 6% (95% CI: 0.8-35%) and 20% (95% CI: 9-39%) for HRD. At 5 years of follow-up, these values increase to 23% (95% CI: 8-57%) and 43% (95% CI: 27-63%), respectively (Fig. 3). The relapse at 5 years of follow-up was 18% (95% CI: 4-58%) for MSD and 25% (95% CI: 10-52%) for HRD (Fig. 4). The overall mortality was 46%. Most of the deaths were transplant-related (15/23 deaths), with infections being the most common cause of death. Other causes of death are summarized in Supplementary Material 3 (www.thejh.org).
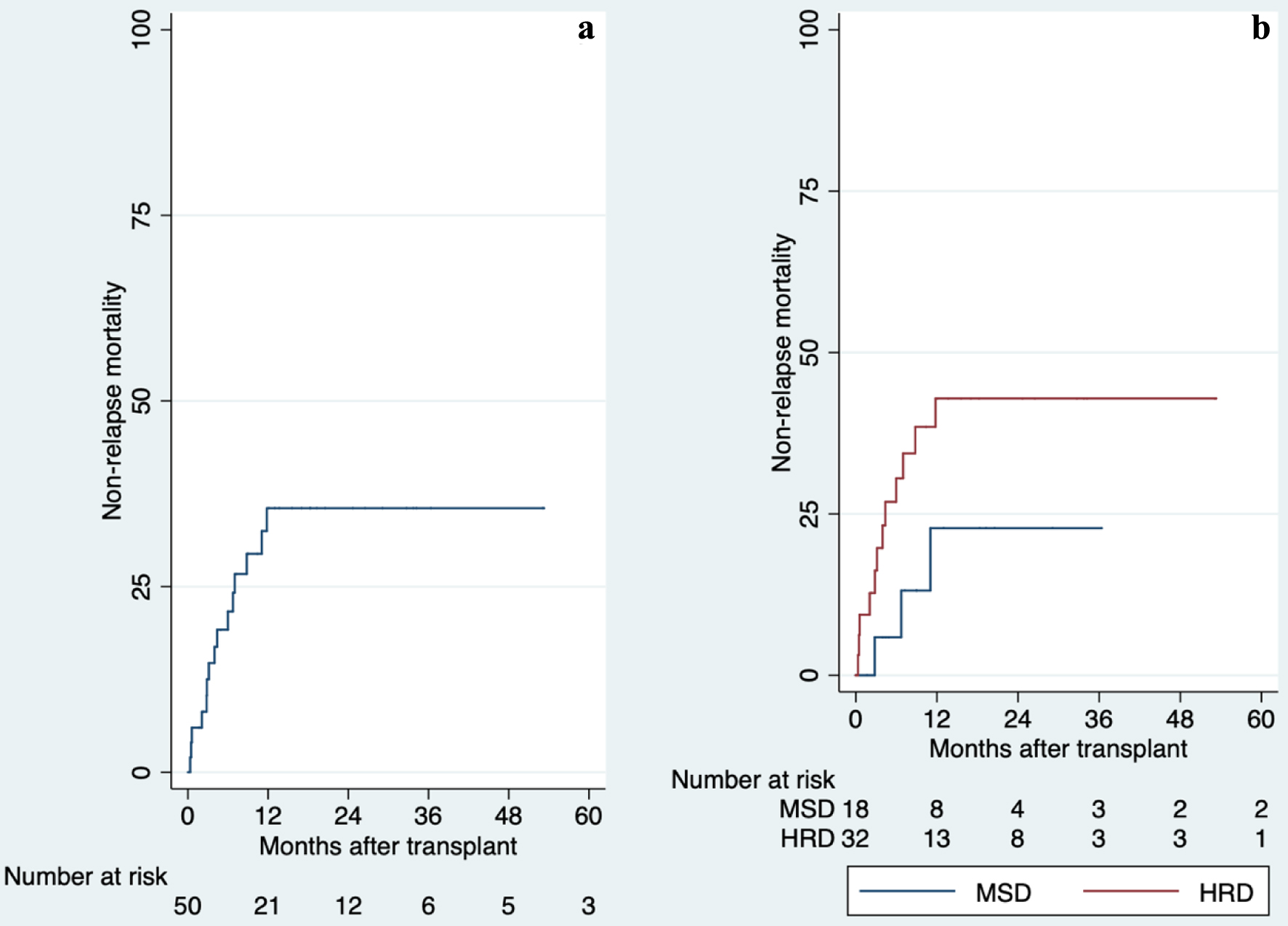 Click for large image | Figure 3. Overall and comparison of non-relapse mortality after allogeneic hematopoietic stem cell transplantation, 2013 - 2019. There is no statistically significant difference between groups (P = 0.190). (a) Overall of non-relapse mortality. (b) Comparison of non-relapse mortality. HRD: haploidentical related donor; MSD: HLA-matched sibling donor. |
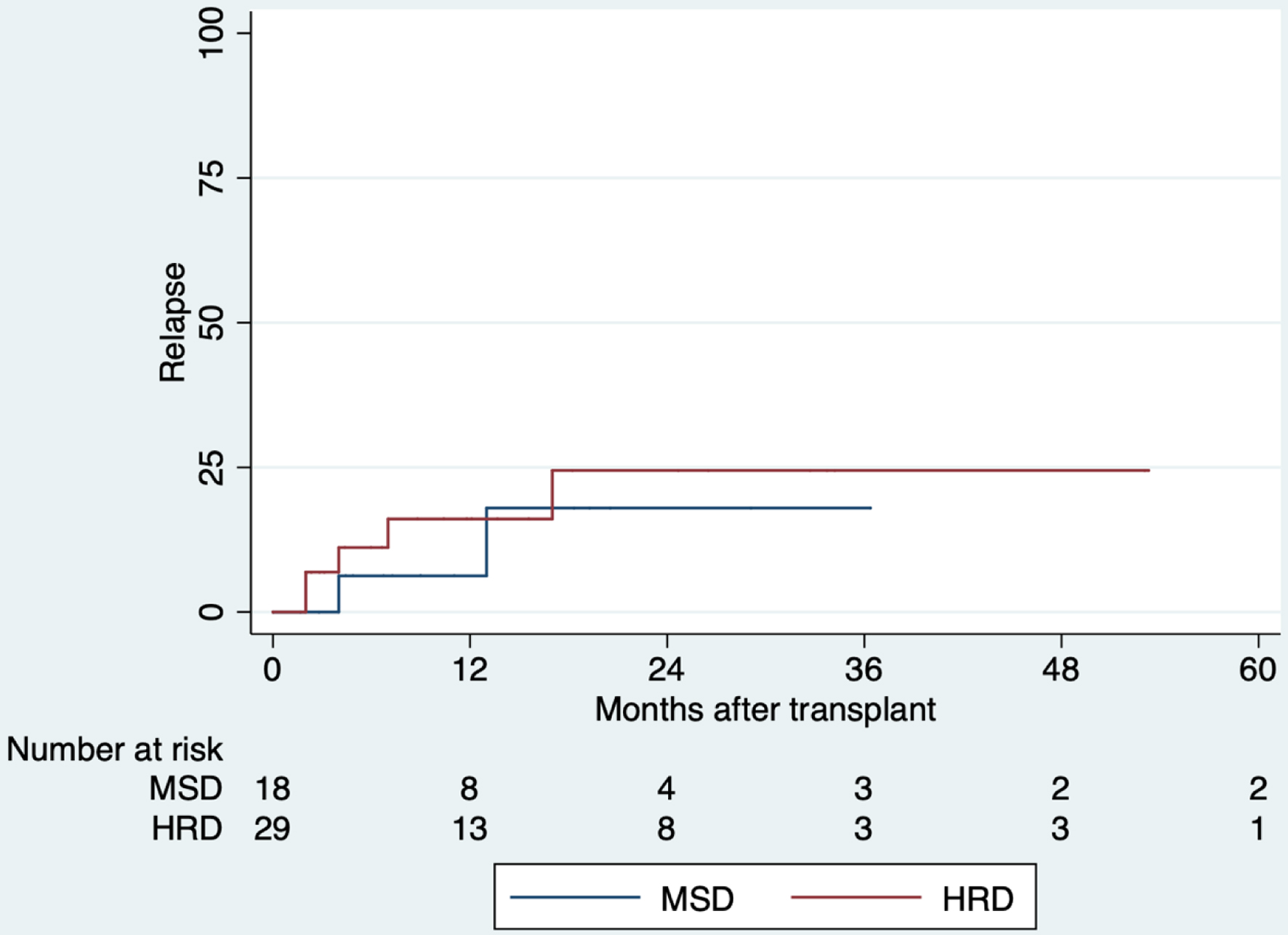 Click for large image | Figure 4. Comparison of relapse after allogeneic hematopoietic stem cell transplantation, 2013 - 2019. There is no statistically significant difference between groups (P = 0.537). HRD: haploidentical related donor; MSD: HLA-matched sibling donor. |
The cumulative incidence of grade II-IV, III-IV acute GvHD and severe chronic GvHD was 44%, 11% and 12% for MSD, and 43%, 9% and 0% for HRD (Table 2).
A total of 12 studies were reviewed and considered relevant to the topic of this paper after literature review [20-31]. Four were from Latin America and four from Europe. Table 3 summarizes the main findings of the studies.
 Click to view | Table 3. Literature Review of Haploidentical Related Donors and HLA-Matched Sibling Donors Transplants in Acute Myeloid Leukemia |
| Discussion | ▴Top |
This study evaluated the clinical outcomes of patients with AML who underwent HRD vs. MSD at a single center in Cali, Colombia. The findings demonstrate that HRD transplantation offers acceptable outcomes, with effectiveness and toxicity rates comparable to established standards. Importantly, HRD provides a valuable treatment option for AML patients who would otherwise face unfavorable outcomes without transplantation.
Donor selection is a crucial factor in determining the success of HSCT for AML. MSD is generally considered the optimal donor source for allogeneic HSCT. However, when MSD is not available, the EBMT-Acute Leukemia Working Party (ALWP) suggested MUD as the subsequent choice due to superiority for OS, leukemia-free survival and NRM shown in certain cohorts [32, 33]. The likelihood of finding a highly matched unrelated donor depends on the patient’s HLA haplotype frequencies and ethnic background. In cases where MSD is unavailable or unsuitable, HLA-mismatched unrelated donors (MM-URDs), UCB and HRD may be considered as alternative options [34].
Our OS and RFS are consistent for MSD and HRD, with those reported in similar Latin American, North American, European and Asian populations [20-23, 25-31] (Table 3). Differences in these outcomes between MSD and HRD were not statistically significant. However, MSD had longer OS and RFS than HRD. This could be explained as follows: first, MSDs are more likely to be HLA-matched to the recipient. However, the MSD availability varies substantially based on ethnicity and age, with rates ranging from 13% to 51% [35]. HLA matching is important for reducing the risk of GvHD, which is a major complication of allogeneic HSCT [36]; second, although there were no differences in donor age between groups, the median age was lower in HRD. Younger and healthier donors can engraft and function effectively in the recipient [37]. Despite these ongoing discussions, EBMT centers’ utilization of HRD has increased by 250% since 2010 for both malignant and non-malignant disorders [34].
In the HRD group, the OS was low, particularly in the low-risk group (EBMT score < 3), with a day-100 NRM reaching 16%, and a high incidence of grade II-IV acute GvHD reaching 46%. These poor outcomes could be explained by three limitations of this study: the small sample size, the single-center design and the absence of long-term follow-up data.
Despite scarcity of prospective studies, some authors of comparative non-randomized trials using PT-Cy and other methods for in vivo selective T-cell-depletion HRD allogeneic HSCT have suggested favorable clinical outcomes (OS, GvHD, RFS, EFS and NRM) comparable to MSD allogeneic HSCT [38-40]. In our center, MUD transplantations are not performed due to issues related to the health system and economic issues related to the logistics of bringing hematopoietic progenitors from abroad.
Initial attempts at haploidentical HSCT were associated with high rates of GvHD [41]. Our study found a 43% of acute GvHD, with only 9% of cases classified as grade III-IV acute GvHD in the HRD group and 11% in the MSD group. Several studies have shown that the risk of acute GvHD is lower in HRD with PT-Cy compared to MSD [28, 42, 43]. Another concern related to haploidentical HSCT is the time to engraftment. Engraftment for neutrophils and platelets in our study took a median of 18 and 22 days, respectively. These results are consistent with findings from other studies [21, 23, 30].
The role of MAC is to eliminate residual hematological disease from the bone marrow, create space for donor stem cells in the host bone marrow, and provide immunosuppression to ensure engraftment. Comparing MAC regimens is complex due to patient and donor variables that influence toxicity and relapse risk. The BUFLU TBI-400 conditioning was used in the majority of cases. Fludarabine synergizes with busulfan by inhibiting DNA ligase and DNA primase, preventing DNA polymerization, and impairing alkylator-induced damage repair [44, 45]. This conditioning regimen has been widely used in other centers [31, 46] and has been associated with lower toxicities [47].
NRM was higher in HRD group as in previous studies [22, 23, 25, 27, 30, 31]. Although it has been proposed that NRM increased with donors other than an HLA-identical sibling donor [48], studies involving alternative donors have never shown NRM rates higher than 40% at 24 months [22, 23, 27, 29]. Additionally, NRM decreased with reduced-intensity conditioning in all risk categories [48]. Retrospective studies have included a non-negligible percentage of non-MAC [20, 22-24, 27]. Furthermore, some of these studies used only chemotherapy as MAC or included TBI in a low percentage of their patients [20, 21, 23, 27]. Our center has always used MAC with TBI. This could explain why 71% of deaths in the HRD group were transplant-related causes (most of them due to infections). In addition, it could also justify our low relapse rates.
Multiple factors hinder the optimal utilization of allogeneic PBSCT in adult patients with AML in our country. There are still limitations in our context to identify patients who benefit of this strategy in the most common scenario such as CR1 because many patients came from other hospitals without adequate or availability molecular and/or cytogenetic tests. Unfortunately, not all hospitals have the logistics and tools to make an adequate prognosis at the onset of the disease. Proof of this is the fact that almost half of individuals included in the study had a low EBMT risk score, and just 25% were classified as high risk. However, outcomes such as NRM seem to correspond to populations that generally would have higher EBMT scores. Consequently, additional elements such as risk stratification by genetics, minimal residual disease, transplant technology enhancements and adequate cytogenetics could improve transplant decisions on the basis of an individualized assessment of both the predicted risk of disease relapse and maximal tolerated NRM for our patients [49-51].
Limitations and strengths
The results of this study should be interpreted in the context of their design. First, it was conducted retrospectively at a single center, limiting the sample size and implying selection and information biases. Secondly, we had a small sample size of allogeneic PBSCT. Third, MUD and UCB transplantation are not performed in the country because these procedures are expensive for the health system and the supplies must be brought out of the country, which makes its implementation difficult. Fourth, the lack of long-term follow-up could affect the estimates of the study times and clinical outcomes.
Despite the limitations, these findings contribute to the better knowledge and characterization of MSD and HRD transplants in AML patients because we described the clinical scenario and outcomes in a low-to-middle income country. Previous studies in Latin American countries have limited data related with engraftment, GvHD, RFS, NRM and relapse. These findings are useful for the medical community that handles patients with similar characteristics in the country and region. Future research should be considered multicenter design with larger sample sizes, randomized, prospective, to compare long-term outcomes of patients who undergo HRD vs. MSD HSCT and new therapeutic strategies to reduce risk of GvHD and other complications of allogenic HSCT.
Conclusions
MSD remains the first choice for allogeneic PBSCT in adult patients with AML in CR1 when available. HRD is still the next option among alternative donors. There is a need to identify strategies that improve outcomes that significantly impact the quality of allogeneic PBSCT and prognosis. Comparative, randomized, prospective studies with longer follow-up are needed to establish the role of haploidentical allogeneic PBSCT among alternative donors.
| Supplementary Material | ▴Top |
Suppl 1. Frequency of hematopoietic stem cell transplantation in Fundacion Valle del Lili, period 2012 - 2020.
Suppl 2. Comparison of overall survival of hematopoietic stem cell transplantation by conditioning regimens, 2013 - 2019.
Suppl 3. Death causes of patients included in the study.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
As this is a retrospective study, patient informed consent was not required.
Author Contributions
Baena JC, Arrieta E, Jaramillo FJ, Manzi E, and Rosales JD participated at conceptualization; Baena JC, Arrieta E, and Manzi E planned the methodology; Rosales MC, Estacio M, Hidalgo A, and Parra-Lara LG did the validation; Baena JC, Arrieta E, Manzi E, and Parra-Lara LG performed the formal analysis; all authors prepared the original draft, Manzi E and Parra- Lara LG reviewed and edited the first draft; Rosales JD performed the project supervision. All authors made significant contributions to writing the manuscript, read and approved the final manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
doi pubmed - Sasaki K, Ravandi F, Kadia TM, DiNardo CD, Short NJ, Borthakur G, Jabbour E, et al. De novo acute myeloid leukemia: A population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980 to 2017. Cancer. 2021;127(12):2049-2061.
doi pubmed - Loke J, Malladi R, Moss P, Craddock C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: a triumph of hope and experience. Br J Haematol. 2020;188(1):129-146.
doi pubmed pmc - Ayuk F, Balduzzi A. Donor Selection for Adults and Pediatrics. In: Carreras E, Dufour C, Mohty M, Kroger N, eds. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies. 7th ed. Cham (CH), 2019; 87-97.
doi pubmed pmc - World Marrow Donor Association (WMDA). Statistics Search & Match Service. 2023 [cited Jun 13, 2023]. Total number of donors and cord blood units. Available from: https://statistics.wmda.info/.
- Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, Hartzman R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339-348.
doi pubmed pmc - Buck K, Wadsworth K, Setterholm M, Maiers M, Confer D, Hartzman R, Schmidt A, et al. High-resolution match rate of 7/8 and 9/10 or better for the be the match unrelated donor registry. Biol Blood Marrow Transplant. 2016;22(4):759-763.
doi pubmed - Majhail NS, Omondi NA, Denzen E, Murphy EA, Rizzo JD. Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2010;16(8):1070-1075.
doi pubmed pmc - Passweg JR, Baldomero H, Peters C, Gaspar HB, Cesaro S, Dreger P, Duarte RF, et al. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant. 2014;49(6):744-750.
doi pubmed pmc - Fuchs EJ. Related haploidentical donors are a better choice than matched unrelated donors: Point. Blood Adv. 2017;1(6):397-400.
doi pubmed pmc - Jaimovich G, Martinez Rolon J, Baldomero H, Rivas M, Hanesman I, Bouzas L, Bonfim C, et al. Latin America: the next region for haematopoietic transplant progress. Bone Marrow Transplant. 2017;52(5):671-677.
doi pubmed - Gale RP, Seber A, Bonfim C, Pasquini M. Haematopoietic cell transplants in Latin America. Bone Marrow Transplant. 2016;51(7):898-905.
doi pubmed pmc - Escobar Morales G, Perilla Galvis DM. Departamento Administrativo de Planeacion. Cali en cifras. Departamen. Cali; 2020. p. 1-220
- Bravo LE, Garcia LS, Collazos P, Carrascal E, Ardila EKG, Millan E, Holguin J. Cancer epidemiology in Cali, 60 years of experience. Colomb Med (Cali). 2022;53(1):e2005050.
doi pubmed pmc - Instituto Nacional de Salud (INS). Direccion de redes de salud publica. 2022. p. 1 Red Nacional de Donacion y Trasplante de Organos y Tejidos.
- Instituto Nacional de Salud (INS). Informe anual red de donacion y trasplantes [Internet]. Bogota D.C.; 2012. [cited Nov 12, 2023]. Available from: https://www.ins.gov.co/Direcciones/RedesSaludPublica/DonacionOrganosYTejidos/Estadisticas/Informe_Red%20donacion%20y%20trasplantes%20Vol2_2012.pdf.
- Iacobelli S, Committee ES. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2013;48(Suppl 1):S1-37.
doi pubmed - Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295-304.
doi pubmed - Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T, Dini G, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer. 2009;115(20):4715-4726.
doi pubmed - Abello V, Rosales C, Pedraza E, Esguerra HJ, Rosales ML, Linares A. 103: Hematopoietic stem cell transplantation (SCT): a single center experience in Colombia. Biology of Blood and Marrow Transplantation [Internet]. 2008;14(2):40.
doi - Leon Rodriguez E, Rivera Franco MM. Outcomes of hematopoietic stem cell transplantation at a limited-resource center in mexico are comparable to those in developed countries. Biol Blood Marrow Transplant. 2017;23(11):1998-2003.
doi pubmed - Trucco JII, Berro M, Rivas MM, Longo PG, Garcia PA, Garcia JJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in argentina: results comparing matched related, unrelated or haploidentical donors. A Grupo Argentino De Trasplante De Medula Osea (GATMO) experience. Blood [Internet]. 2017;130(1):5558.
doi - Versluis J, Labopin M, Ruggeri A, Socie G, Wu D, Volin L, Blaise D, et al. Alternative donors for allogeneic hematopoietic stem cell transplantation in poor-risk AML in CR1. Blood Adv. 2017;1(7):477-485.
doi pubmed pmc - Ostgard LS, Norgaard JM, Sengelov H, Severinsen M, Friis LS, Marcher CW, Dufva IH, et al. Comorbidity and performance status in acute myeloid leukemia patients: a nation-wide population-based cohort study. Leukemia. 2015;29(3):548-555.
doi pubmed - Salvatore D, Labopin M, Ruggeri A, Battipaglia G, Ghavamzadeh A, Ciceri F, Blaise D, et al. Outcomes of hematopoietic stem cell transplantation from unmanipulated haploidentical versus matched sibling donor in patients with acute myeloid leukemia in first complete remission with intermediate or high-risk cytogenetics: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2018;103(8):1317-1328.
doi pubmed pmc - Liu Y, Huang X, Fei Q, Xu L, Zhang X, Liu K, Chen Y, et al. Comparison analysis between haplo identical stem cell transplantation and matched sibling donor stem cell transplantation for high-risk acute myeloid leukemia in first complete remission. Sci China Life Sci. 2019;62(5):691-697.
doi pubmed - Rashidi A, Hamadani M, Zhang MJ, Wang HL, Abdel-Azim H, Aljurf M, Assal A, et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019;3(12):1826-1836.
doi pubmed pmc - Figueroa J, Pedraza E, Pena O, Mora E, Ardila S, Guarin A, et al. Comparison between allogeneic stem cell transplantation from unmanipulated haploidentical, unrelated and matched related donors for acute lymphoblastic leukemia and acute myeloid leukemia: experience in a single center in Colombia. Biology of Blood and Marrow Transplantation [Internet]. 2020;26(3):S293-S294.
doi - Yanada M, Takami A, Yamasaki S, Arai Y, Konuma T, Uchida N, Najima Y, et al. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia conducted in Japan during the past quarter century. Ann Hematol. 2020;99(6):1351-1360.
doi pubmed - Mehta RS, Saliba RM, Ghanem S, Alousi AM, Rondon G, Anderlini P, Al-Atrash G, et al. Haploidentical versus matched unrelated versus matched sibling donor hematopoietic cell transplantation with post-transplantation cyclophosphamide. Transplant Cell Ther. 2022;28(7):395.e1-395.e11.
doi pubmed pmc - Rieger MJ, Stolz SM, Muller AM, Schwotzer R, Nair G, Schneidawind D, Manz MG, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched related and unrelated donor transplant in acute myeloid leukemia and myelodysplastic neoplasm. Bone Marrow Transplant. 2023;58(10):1121-1129.
doi pubmed pmc - Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828.
pubmed - Piemontese S, Ciceri F, Labopin M, Arcese W, Kyrcz-Krzemien S, Santarone S, Huang H, et al. A comparison between allogeneic stem cell transplantation from unmanipulated haploidentical and unrelated donors in acute leukemia. J Hematol Oncol. 2017;10(1):24.
doi pubmed pmc - Lee CJ, Savani BN, Mohty M, Labopin M, Ruggeri A, Schmid C, Baron F, et al. Haploidentical hematopoietic cell transplantation for adult acute myeloid leukemia: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102(11):1810-1822.
doi pubmed pmc - Besse K, Maiers M, Confer D, Albrecht M. On modeling human leukocyte antigen-identical sibling match probability for allogeneic hematopoietic cell transplantation: estimating the need for an unrelated donor source. Biol Blood Marrow Transplant. 2016;22(3):410-417.
doi pubmed - Kanda J. Effect of HLA mismatch on acute graft-versus-host disease. Int J Hematol. 2013;98(3):300-308.
doi pubmed - Kollman C, Spellman SR, Zhang MJ, Hassebroek A, Anasetti C, Antin JH, Champlin RE, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127(2):260-267.
doi pubmed pmc - Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, Armand P, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033-1040.
doi pubmed pmc - Bashey A, Zhang X, Jackson K, Brown S, Ridgeway M, Solh M, Morris LE, et al. Comparison of outcomes of hematopoietic cell transplants from t-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-identical sibling donors: a multivariable analysis including disease risk index. Biol Blood Marrow Transplant. 2016;22(1):125-133.
doi pubmed - Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, Pingali SR, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014;20(12):1975-1981.
doi pubmed pmc - Handgretinger R, Lang P. The history and future prospective of haplo-identical stem cell transplantation. Cytotherapy. 2008;10(5):443-451.
doi pubmed - McCurdy SR, Kanakry CG, Kasamon YL, Bolanos Meade J, Tsai HL, Showel MM, et al. Graft-versus-host disease (GVHD) and survival outcomes after HLA-haploidentical (Haplo) bone marrow transplant (BMT) compare favorably with matched related donor (MRD), and matched unrelated donor (MUD) BMT utilizing high-dose posttransplantation cyclophosphamide (PTCy). Blood. 2014;124(21):730.
- Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, Bregante S, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20(10):1573-1579.
doi pubmed - Brusini P, Salvetat ML, Parisi L, Zeppieri M, Tosoni C. Discrimination between normal and early glaucomatous eyes with scanning laser polarimeter with fixed and variable corneal compensator settings. Eur J Ophthalmol. 2005;15(4):468-476.
doi pubmed - de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, Shpall EJ, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104(3):857-864.
doi pubmed - Kanakry CG, O'Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, Mielcarek M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497-3505.
doi pubmed pmc - Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie D, Zhang Y, et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol Oncol. 2013;6:15.
doi pubmed pmc - Gratwohl A. The EBMT risk score. Bone Marrow Transplant. 2012;47(6):749-756.
doi pubmed - Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70.
doi pubmed - Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J, Grimwade D, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131(12):1275-1291.
doi pubmed pmc - Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


