| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 12, Number 6, December 2023, pages 243-254
Impact of Cytogenetic Abnormalities, Induction and Maintenance Regimens on Outcomes After High-Dose Chemotherapy and Autologous Stem Cell Transplantation in Patients With Newly Diagnosed Multiple Myeloma: A Decade-Long Real-World Experience
Aswani Thurlapatia, d, William Wessonb, d, James A. Davisc, Kelly J. Gaffneyc, Erin Weedac, Arash Velayatia, Jonathan K. Bakosa, Katelynn Grangerc, Deidra Smithc, Andy P. Maldonadoc, Taylor Herringtonc, Julia Pottsa, Hamza Hashmia, e
aDepartment of Hematology and Bone Marrow Transplant, Medical University of South Carolina, Hollings Cancer Center, Charleston, SC 29425, USA
bUniversity of Kansas School of Medicine, Kansas City, KS 66103, USA
cMedical University of South Carolina College of Pharmacy, Charleston, SC 29425, USA
dThese authors contributed equally to the creation of the manuscript.
eCorresponding Author: Hamza Hashmi, Division of Hematology/Oncology, Medical University of South Carolina, Charleston, SC 29425, USA
Manuscript submitted October 3, 2023, accepted November 22, 2023, published online December 9, 2023
Short title: HRCAs on Outcomes After HDT-ASCT for NDMM
doi: https://doi.org/10.14740/jh1201
| Abstract | ▴Top |
Background: High-dose chemotherapy and autologous stem cell transplant (HDT-ASCT) has become a standard of care for transplant eligible newly diagnosed multiple myeloma (NDMM) patients. While cytogenetic abnormalities have been shown to affect outcomes after HDT-ASCT in clinical trials, these trials often exclude or underrepresent elderly patients with comorbidities and those belonging to ethnic minorities. We describe our institutional experience highlighting the impact of high-risk cytogenetic abnormalities (HRCAs) on outcomes after HDT-ASCT for NDMM patients.
Methods: A total of 449 patients with NDMM who underwent HDT-ASCT between February 2012 and August 2022 were included in this retrospective analysis. HRCAs included the presence of one or more of: deletion 17p, t(14;16), t(4;14), and amplification 1q. Survival analyses, including progression-free survival (PFS) and overall survival (OS), were performed using Kaplan-Meier estimator.
Results: With a median follow-up of 29 (1 - 128) months for the entire patient population, the best overall response rate for the patients with HRCAs was lower compared to those with standard risk cytogenetics (90% vs. 96%; P = 0.01). Patients with HRCAs had an inferior PFS compared to patients with standard-risk cytogenetics (29 vs. 58 months; P < 0.001) without a difference in OS (70 months vs. not reached; P = 0.13).
Conclusions: In a multivariable analysis adjusting for factors including age, race, and comorbidities, HRCAs, non-lenalidomide-based maintenance, non-proteasome inhibitor-based maintenance, and age greater than 65 were associated with inferior PFS. Amongst these factors, only non-lenalidomide-based maintenance was associated with inferior OS.
Keywords: Myeloma; High-dose therapy; Transplant; High risk; Cytogenetics; Lenalidomide; Proteasome inhibitor
| Introduction | ▴Top |
In transplant eligible newly diagnosed multiple myeloma (NDMM) patients, the combination of triplet or quadruplet induction chemotherapy followed by high-dose chemotherapy and autologous stem cell transplant (HDT-ASCT) with subsequent maintenance therapy has become the standard of care. The Intergroupe Francophone du Myelome (IFM) 2009 prospective study showed a significantly superior progression-free survival (PFS) with triplet induction, upfront ASCT followed by 1 year of maintenance lenalidomide (47.3 vs. 35 months) [1]. However, there was no overall survival (OS) benefit seen with upfront ASCT after a median follow-up of 8 years. The DETERMINATION trial, a phase III randomized controlled trial comparing triplet induction followed by early versus delayed ASCT for patients with NDMM revealed similar results with the upfront ASCT and prolonged maintenance lenalidomide (till progression), leading to a 21.3-month benefit in the median PFS but no improvement in OS [2]. Subsequent clinical trials have also shown a similar survival advantage with the addition of an anti-CD38 monoclonal antibody to a triplet backbone and use of second-generation proteasome inhibitors (PIs) leading to improved depth and duration of remission with upfront HDT-ASCT [3, 4]. The subgroup analyses of the GRIFFIN and FORTE trials indicated the presence of high-risk cytogenetic abnormalities (HRCAs) as one of the most important clinical factors determining the treatment outcomes in NDMM [5]. While Perrot et al developed the cytogenetic prognostic index based on the presence of certain cytogenetics, the definition of high-risk disease biology continues to evolve and varies amongst clinical trials [6]. These clinical trials underrepresent ethnic minorities and often exclude patients due to age, frailty, comorbidities, and organ dysfunction that otherwise could receive and benefit from ASCT. Hence, there remains a need to determine whether cytogenetic abnormalities impact efficacy outcomes after HDT-ASCT for NDMM in a real-world setting with many patients who would not have met eligibility criteria used in clinical trials. In this study, we describe a single-center 10-year experience highlighting the impact of cytogenetic risk on outcomes after upfront HDT-ASCT in a population enriched for the elderly (> 65 years), non-Hispanic Black, and renally impaired patients.
| Materials and Methods | ▴Top |
We conducted a retrospective analysis of all adult patients with NDMM who received induction chemotherapy followed by HDT-ASCT and maintenance chemotherapy between February 2012 and August 2022 at the Medical University of South Carolina. Institutional review board approval was obtained, and the study was conducted according to the Declaration of Helsinki. Patients were included if they had NDMM and had undergone induction chemotherapy followed by HDT-ASCT with day +100 response available at the time of data cutoff. Renal impairment was defined as serum creatinine > 2 mg/dL or creatinine clearance < 60 mL/min/1.73 m2. Prior to ASCT, patients received one-time intravenous (IV) infusion of melphalan given at a dose of either 200 mg/m2 or 140 mg/m2. ASCT was performed either in the inpatient or outpatient setting based on physician discretion. Supportive care and infectious prophylaxis were administered as per institutional guidelines and standard operating procedures. Patients initiated maintenance chemotherapy at or around day +100 post ASCT. The choices of induction and maintenance regimens, transplant eligibility, and melphalan dosing (standard or reduced) were physician dependent. Relevant data pertaining to patient demographics, disease characteristics, treatment history, and safety and efficacy outcomes were extracted by investigators from the electronic health record. Based on the type of cytogenetic abnormalities, patients were stratified as having high-risk or standard-risk cytogenetics. HRCAs included the presence of one or more of: deletion 17p (del 17p), translocation 14;16 (t14;16), translocation 4;14 (t4;14), and amplification 1q (amp 1q) on fluorescent in situ hybridization (FISH) [7]. Gain of 1q (+1q) was considered as a standard risk cytogenetic abnormality. Adverse events were graded based on the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [8]. Responses were assessed based on International Myeloma Working Group (IMWG) response criteria at different time points including prior to ASCT, day +100, and yearly post ASCT [9]. OS was calculated as the time from initiation of induction chemotherapy to death or last contact, and PFS was calculated as the time from initiation of induction chemotherapy to disease progression, death, or last contact.
Data were presented as counts with percentages for categorical variables and medians with ranges for continuous variables. Categorical variables were compared between cohorts using Chi-square or Fisher exact tests, with continuous variables compared using Mann-Whitney U tests. Kaplan-Meier survival curves and log-rank tests were used to examine OS and PFS by cytogenetic abnormalities and induction and maintenance regimens. Cox proportional hazards regression models were used to estimate hazard ratios (HR) and 95% confidence interval (CI) for the association of cytogenetics and type of induction and maintenance chemotherapy with OS, PFS while adjusting for pertinent patient and disease characteristics. A P value < 0.05 was considered statistically significant, all analyses were two-sided, and analyses were conducted using SPSS v28.
| Results | ▴Top |
At the time of data cutoff, a total of 449 consecutive patients with NDMM had received induction chemotherapy followed by HDT-ASCT. The median patient age at the time of diagnosis was 60 (range 25 - 81) years with 35% (156/449) of the study population more than 65 years of age. Forty-two percent of the patients were non-Hispanic Black and 15% of the patients had impaired renal function at the time of ASCT. While 16% had HRCAs and 80% had standard risk cytogenetics, 24% of the patients in the standard-risk group and 44% of the patients in the high-risk group had gain of +1q abnormality. Patients had received a median of five (range 3 - 12) cycles of induction chemotherapy, and nearly 60% of the patients received lenalidomide-based induction. Among patients who received post-transplant maintenance therapy, 67% received lenalidomide-based maintenance, and a majority of these received single agent lenalidomide, while 14% of the patients received lenalidomide in combination with other agents for maintenance. Eleven percent of the study population did not receive post-transplant maintenance due to various reasons such as patient preference, progression of disease, or death. Baseline characteristics of these patients for each of the categories are presented in Table 1.
 Click to view | Table 1. Patient, Disease, and Response Characteristics |
With a median follow-up of 29 months (range 1 - 128), patients with HRCAs had a lower overall response rate compared to patients with standard-risk cytogenetics (90% vs. 96%; P = 0.01). However, rates of very good partial response (VGPR) or better were not clinically or statistically different between the two groups (85% vs. 83%; P = 0.67). The overall response rate (ORR) at day +100 post ASCT were 95% and 88% for the patients with standard and HRCAs, respectively (P = 0.09) (Table 1). Patients with HRCAs had an inferior median PFS compared to patients with standard-risk cytogenetics (29 months vs. 58 months; P < 0.001) (Table 2, Fig. 1a). Similarly, the median OS was 70 months (range 1 - 101) in patients with HRCAs and was not reached (NR) (range 1 - 128) in the standard-risk group; however, this difference was not statistically significant (P = 0.13) (Fig. 1b). Compared to the patients without +1q abnormality, patients with this cytogenetic abnormality had inferior PFS (38 vs. 76 months, P = 0.01) as well as OS (60 vs. NR, P = 0.01) without a difference in best ORR (98% vs. 96%, P = 0.64) and VGPR or better (82% vs. 83%, P = 0.84) rates (Table 2, Fig. 2a, b). Patients who received lenalidomide-based induction therapy had a similar best ORR (96% vs. 94%, P = 0.41) and VGPR or better (84% vs. 83%, P = 0.09) when compared to those who did not receive lenalidomide as part of induction, respectively. However, patients who received lenalidomide-based induction therapy had a superior PFS (53 months, range 1 - 112) as compared to those who did not (39 months, range 1 - 123) (P = 0.05) (Fig. 3a). In addition, the OS was NR (1 - 112) in the lenalidomide-based induction group vs. 78 months (1 - 123) in non-lenalidomide-based induction group (P = 0.02) (Fig. 3b). Patients who received lenalidomide as part of maintenance therapy had a similar best ORR (98% vs. 91%, P < 0.001) and VGPR or better (87% vs. 86%, P = 0.16) when compared to those who did not receive lenalidomide as part of maintenance, respectively. However, patients who received lenalidomide-based maintenance had superior PFS (53 months, range 1 - 123) as compared to those who did not receive lenalidomide (38 months, range 2 - 91) as part of maintenance (P = 0.003) (Fig. 4a). On the contrary, the OS was 104 months in the lenalidomide maintenance group and was NR in the non-lenalidomide group (P = 0.02) (Fig. 4b). Patients who received PI-based maintenance therapy had a numerically better ORR (94% vs. 89%, P = 0.23) and VGPR or better (86% vs. 76%, P = 0.054) when compared to those who did not receive PI as part of maintenance, respectively, but statistical significance was not reached (Table 2). Patients who received PI-based maintenance had numerically better PFS (54 months, range 11 - 97) as compared to those who received non-PI-based maintenance (46 months, range 37 - 55) (P = 0.46) (Fig. 5a). The OS was NR in the PI-based group and was 92 months (range NR) in non-PI group (P = 0.82) (Fig. 5b).
 Click to view | Table 2. Survival Outcomes and Response Rates by Factors of Cytogenetic Risk Profile, Induction, and Maintenance Regimen |
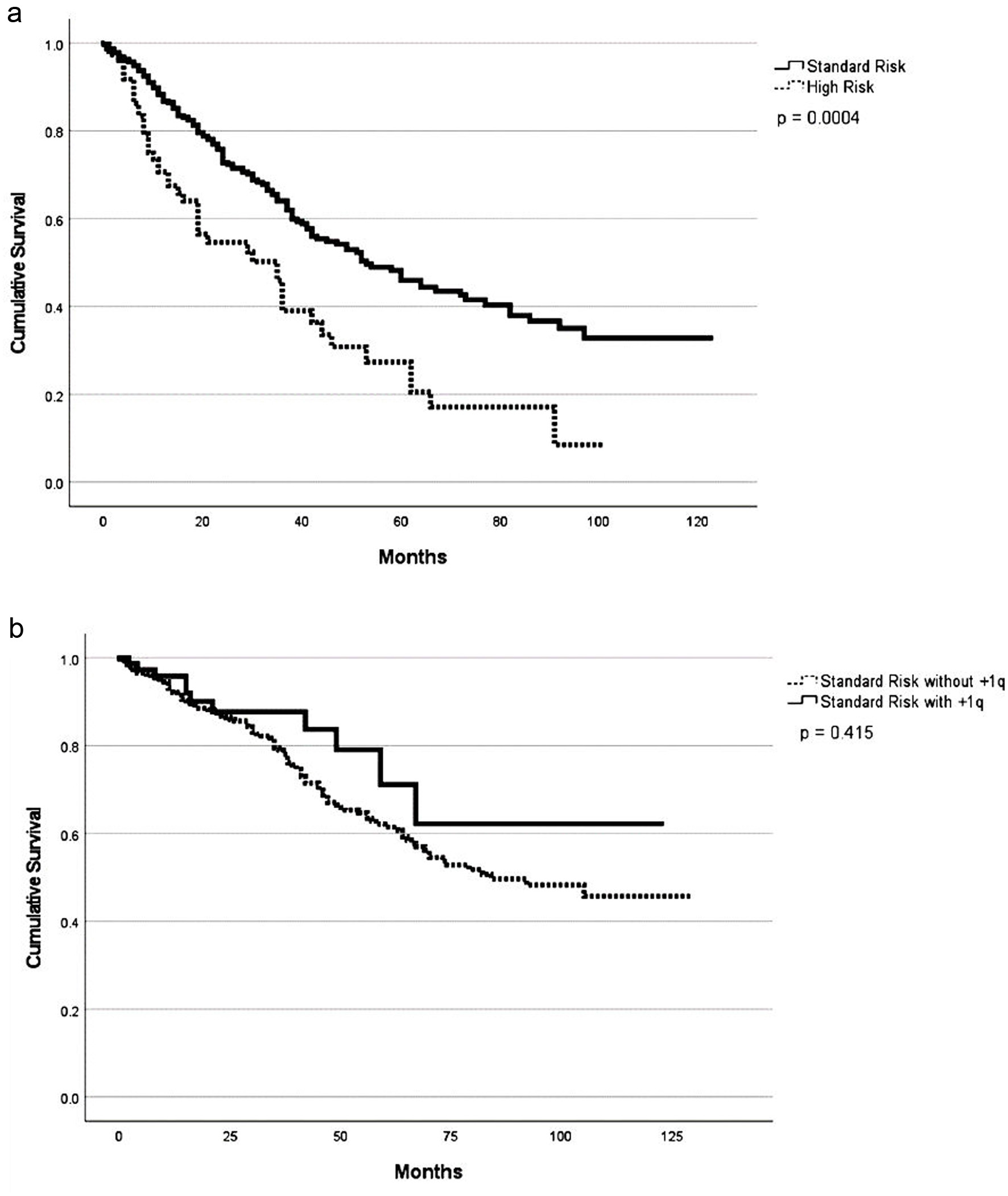 Click for large image | Figure 1. Progression-free survival (a) and overall survival (b) based on cytogenetic profile. |
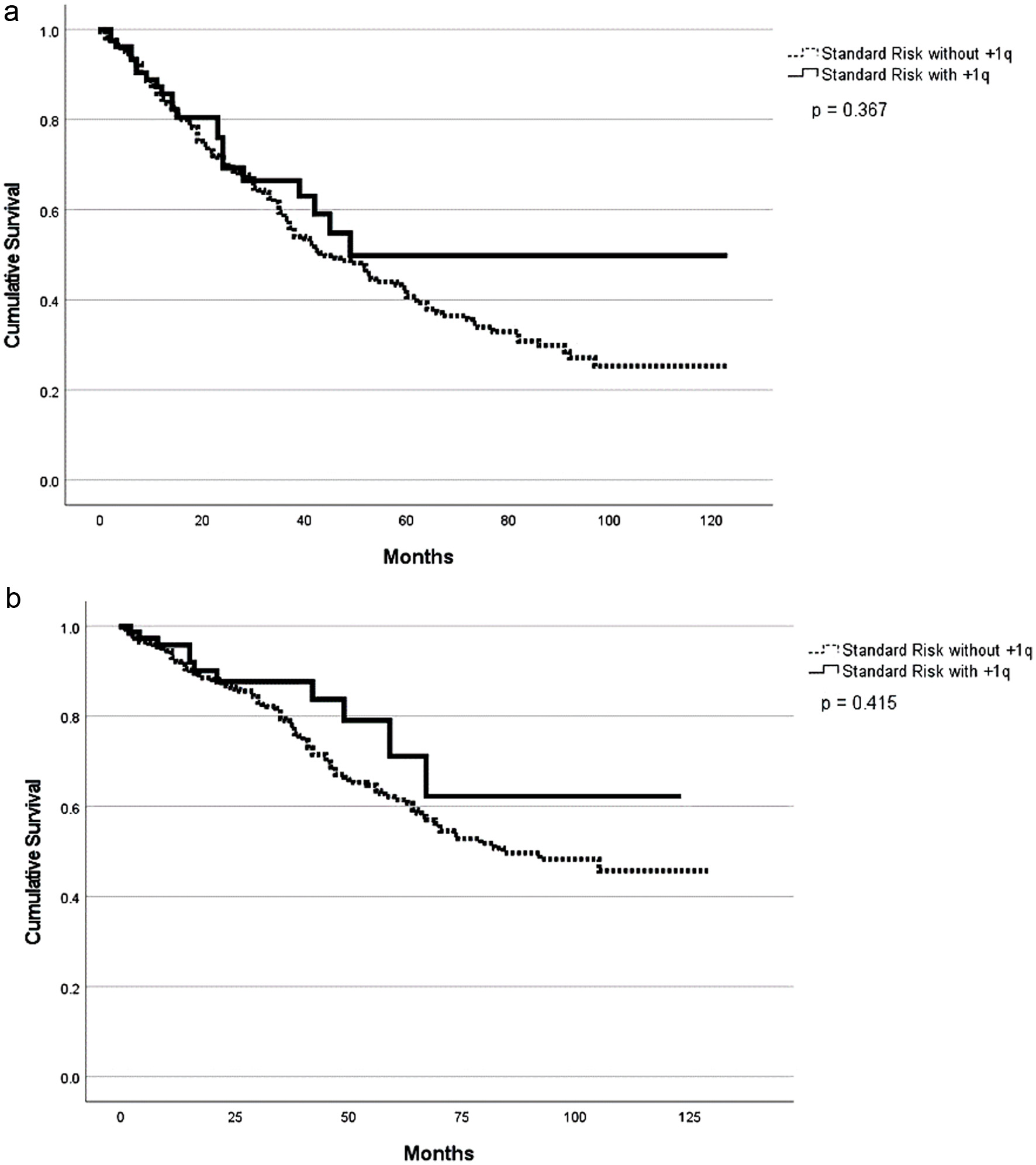 Click for large image | Figure 2. Progression-free survival (a) and overall survival (b) based on presence of +1q abnormality among standard risk. |
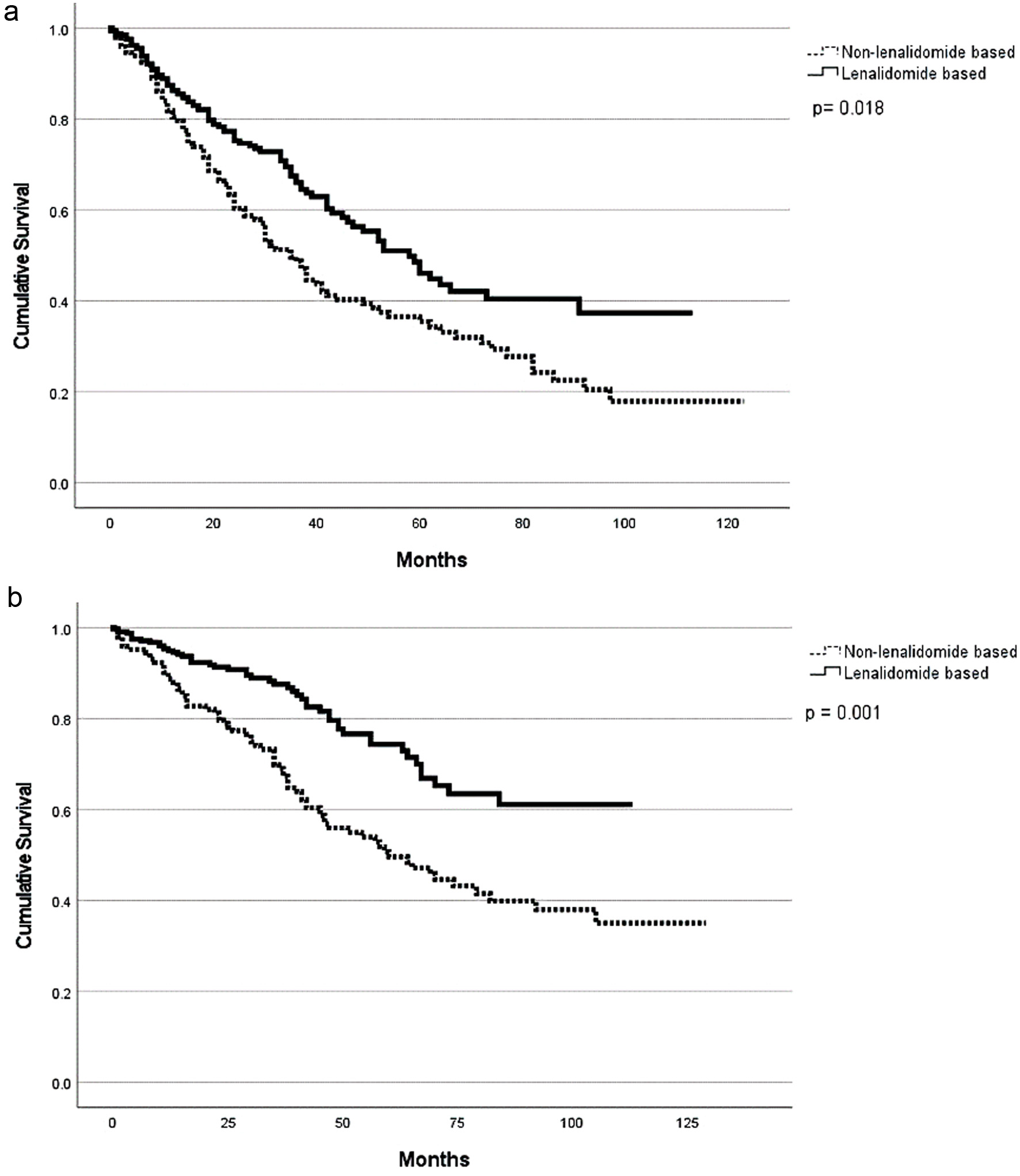 Click for large image | Figure 3. Progression-free survival (a) and overall survival (b) based on lenalidomide-based induction. |
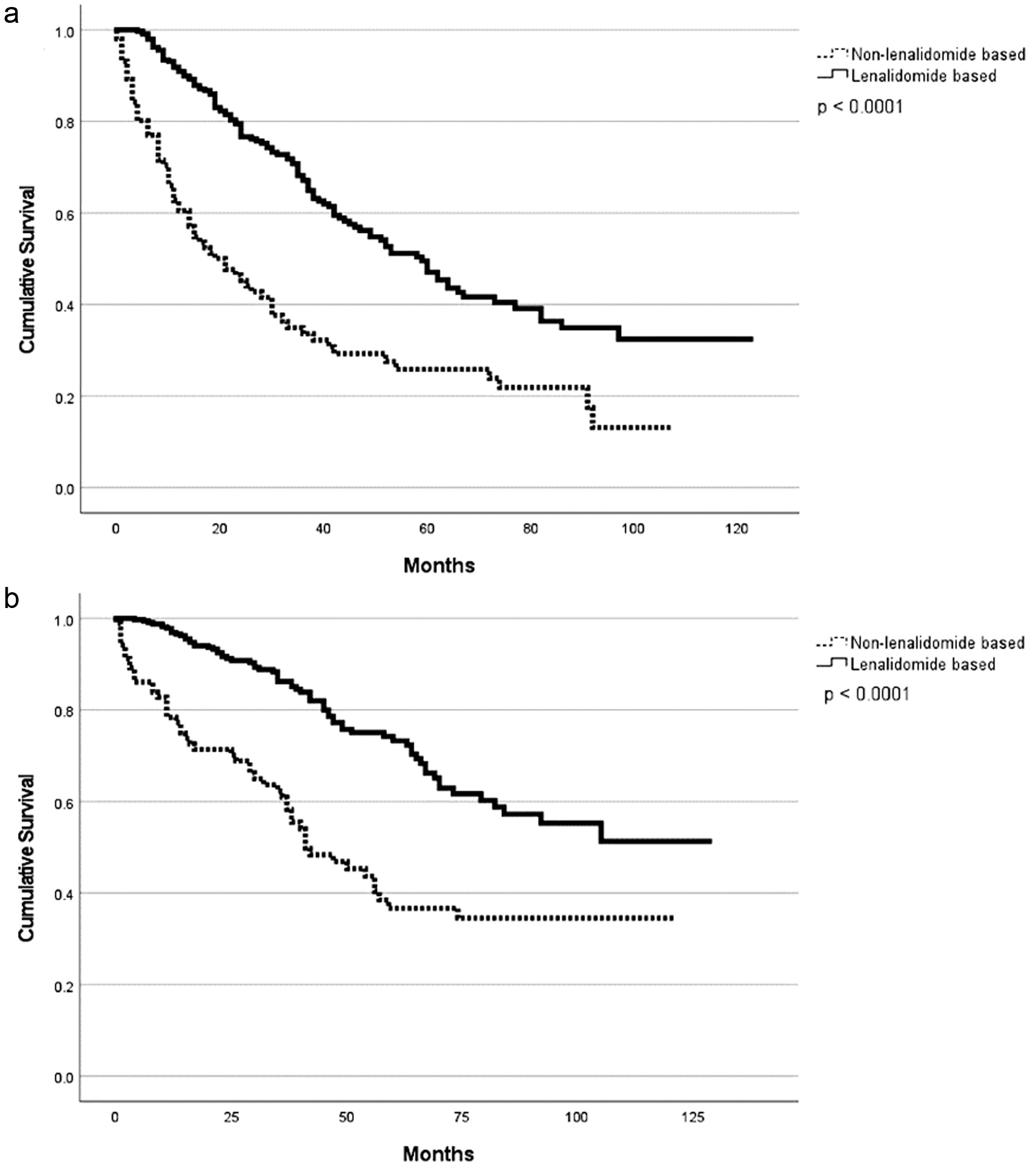 Click for large image | Figure 4. Progression-free survival (a) and overall survival (b) based on lenalidomide-based maintenance. |
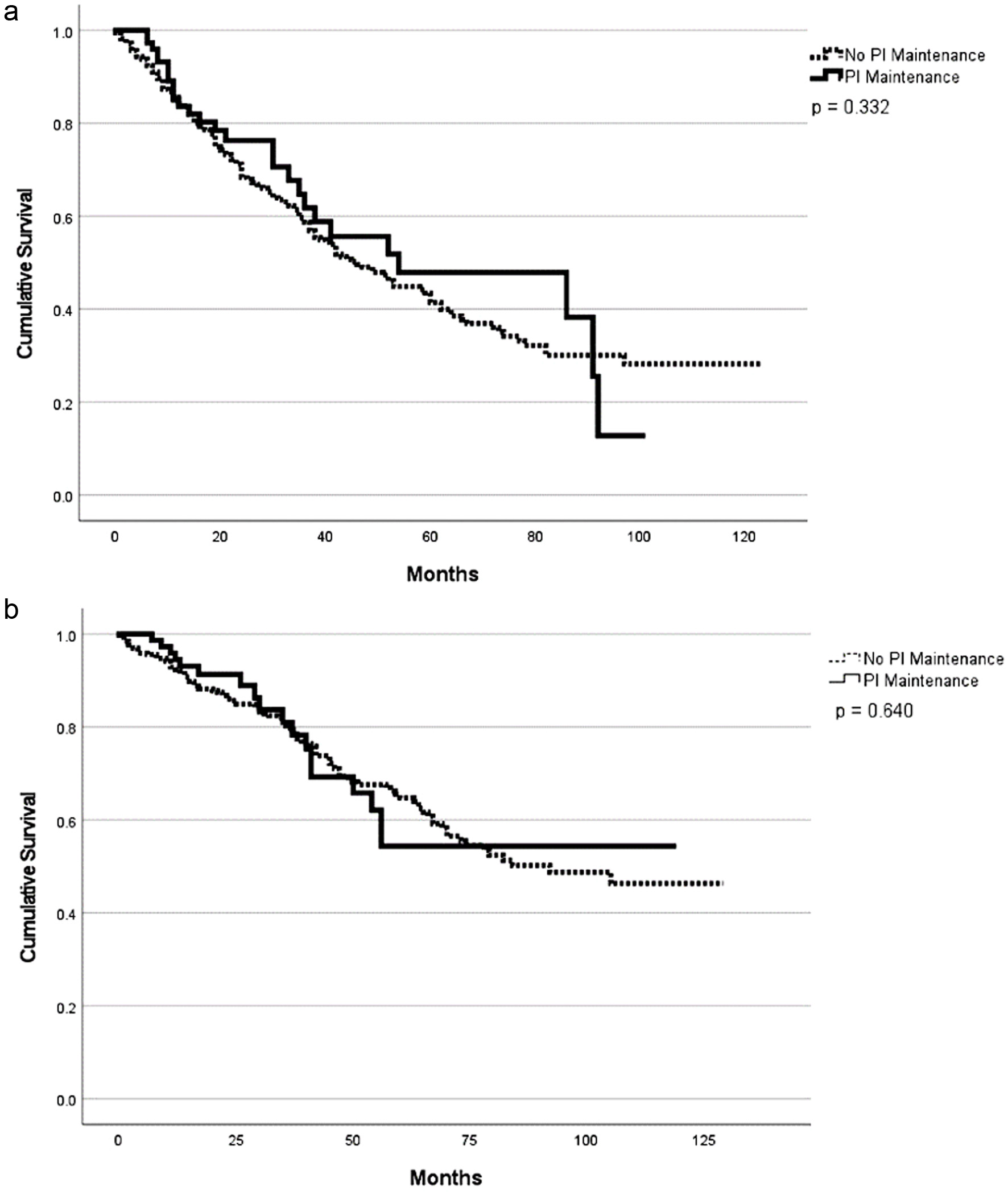 Click for large image | Figure 5. Progression-free survival (a) and overall survival (b) based on proteasome inhibitor vs. non-proteasome inhibitor-based maintenance. |
Individuals with +1q mutation were compared with standard and high-risk cytogenetic profiles. These two groups had no significant differences in baseline characteristics, apart from the cytogenetic risk profiles (Supplementary Material 1, www.thejh.org). The use of chemotherapy induction regimens favored a carfilzomib-based induction in the high-risk group compared to the standard-risk group (P = 0.045). There was an increased PFS (38 months, range 24 - 73) in the standard-risk group compared to the high-risk group (19 months, range 11 - 44) (P = 0.03) with no observed differences in OS (Supplementary Material 2, www.thejh.org).
In the univariate analysis for the entire population, several factors including high-risk cytogenetics, lenalidomide-based induction, and lenalidomide-based maintenance with inferior PFS and OS (Table 3). After adjusting for other factors including patient age, ethnicity, renal dysfunction, disease stage, cytogenetics, melphalan dose, lenalidomide-based induction, lenalidomide-based maintenance, and PI-based maintenance; a multivariate analysis showed high-risk cytogenetic profile was associated with superior PFS (HR 0.29, CI 0.14 - 0.57) but not OS. Age greater than 65 was associated with inferior PFS (HR 2.28, CI 1.16 - 4.47), but not OS. Lenalidomide-based maintenance was associated with both superior PFS (HR 0.27, CI 0.12 - 0.61) and OS (HR 0.16, CI 0.05 - 0.46). PI-based maintenance was associated with superior PFS (HR 0.19, CI 0.07 - 0.51), but not OS (Table 4).
 Click to view | Table 3. Univariate Analysis of Factors Associated With PFS and OS |
 Click to view | Table 4. Multivariate Analysis of Factors Associated With PFS and OS |
| Discussion | ▴Top |
The role of upfront HDT-ASCT to achieve deep and durable remission in patients with NDMM has remained a debatable topic, especially in those with HRCAs. The IFM 2009 study comparing upfront vs. delayed ASCT with limited duration maintenance demonstrated PFS benefit in standard-risk patients with upfront ASCT, but not in individuals with HRCAs. On the contrary, the DETERMINATION trial comparing upfront vs. delayed ASCT with ongoing maintenance revealed significant PFS benefit with upfront ASCT in the NDMM patients with HRCAs. Similarly, the FORTE study showed improvements in PFS with carfilzomib- and lenalidomide-based induction followed by ASCT including patients with one or more HRCAs. However, these landmark trials which have established the role of HDT-ASCT for NDMM utilized different induction and maintenance regimens and excluded certain patient populations including those greater than 65 years of age and patients with comorbidities like impaired renal function. Moreover, ethnic minorities have been underrepresented in these trials which were largely conducted in Europe and the United States. The definition of HRCAs and risk stratification also varied based on contemporary consensus and evolutionary evidence. With analyses of patients with older age (> 65 years), ethnic minorities, and significant renal dysfunction, our study incorporates individuals seen in daily real-world clinical practice who would have otherwise been excluded from clinical trials. Like previously published evidence from randomized controlled trials, our study shows patients with HRCAs remain at a high-risk of disease progression with an inferior PFS. While there was no difference in OS, this could be related to short follow-up, and a difference may emerge with longer follow-up.
In addition to the HRCAs currently incorporated into the Revised International Scoring System (R-ISS), gain of chromosome 1q is a common cytogenetic abnormality (40% of all NDMM patients) which has a debatable impact on prognosis [10]. Earlier studies such as IFM99-02, IFM99-04, CMG2002 trial, and Total Therapy 2 trial (TT2) showed patients with +1q21 had inferior outcomes [11]. However, a large meta-analysis concluded when adjusted for other HRCAs, +1q was not found to be a significant prognostic factor [12]. Hence, as part of our study, we have analyzed the outcomes based on presence of +1q abnormality. Around one-fourth of our study population had +1q abnormality, and about one-third of these were associated with another HRCA. Our study demonstrated individuals with only +1q abnormality and no other HRCAs still had an inferior PFS and OS when compared to individuals without +1q abnormality, thus highlighting the negative prognostic impact of this cytogenetic abnormality. However, in the multivariate analysis adjusting for factors affecting survival outcomes, presence of +1q abnormality was not associated with inferior PFS or OS.
Our study also shows patterns of utilization of induction and maintenance regimens with most patients receiving lenalidomide-based therapies in a real-world setting. Lenalidomide-based maintenance therapy was associated with superior PFS and OS in a multivariate analysis after adjusting for other factors. A majority of patients (about 70%) received lenalidomide for maintenance, either as a single agent or in combination with other agents. The study confirms that compared to no maintenance or observation (11% of the study patients), patients receiving maintenance therapy post ASCT had superior outcomes. Due to the small number of individuals who received lenalidomide in combination with other agents like PI or daratumumab, we do not have statistically significant data comparing single agent lenalidomide maintenance with combination therapies. Similarly, a relatively small proportion of patients (13%) received PI-based maintenance therapies in the HRCAs group which did not show a statistically significant difference in outcomes.
One additional strength of this study is the strong presence of a non-Hispanic Black population which is usually underrepresented in clinical trials. The prevalence of multiple myeloma (MM) is higher in non-Hispanic Black patients when compared to other populations, but given their limited representation in clinical trials, it is difficult to generalize the results of these clinical trials. In our study, non-Hispanic Black patients comprised 42% of the patient population, were diagnosed at a younger age (59 vs. 62 years) and had a lower incidence of HRCAs compared to non-Hispanic White patients (16% vs. 23%), which is consistent with previously published studies [13]. In univariable and multivariable analyses, race/ethnicity was not found to be associated with a statistically significant difference in outcomes, highlighting that non-Hispanic Black patients derive a similar benefit from HDT-ASCT.
Despite multiple merits, there are also several limitations in our study. Although the depth and duration of response with HDT-ASCT as seen in our study are similar to the outcomes reported in clinical trials and other real-world experiences, an inherent limitation of this analysis is its retrospective nature, and hence, the results need to be interpreted with caution. While our study focused on most relevant HRCAs as agreed upon by the IMWG, other risk factors including presence of circulating plasma cells, extramedullary disease, and gene expression profiling that impact outcomes in NDMM were not studied in our analysis. We only included patients referred for transplant consultation and eventually underwent ASCT at our center. While there is a fair representation of the elderly, non-Hispanic Black, and renally impaired patient populations, the study does not include patients who were deemed ineligible for ASCT due to other barriers like frailty, poor performance status, and other comorbidities. Hence, there is some degree of selection bias for the physiologically fit patients undergoing ASCT. Moreover, response assessments were only performed at specific time points for Center for International Blood and Marrow Transplant Research (CIBMTR) reporting (pre-ASCT, day +100, and yearly after ASCT); hence, these response assessments are limited when compared with the prospective setting of a clinical trial. Many of the patients received induction and maintenance chemotherapy with community providers, hence, data on safety of these regimens were not available. The choices of induction and maintenance regimens, transplant eligibility, and melphalan dosing (standard or reduced) were physician dependent and the underlying rationale for physicians’ decision could not be accurately determined due to the retrospective nature of the study. Minimal residual disease (MRD) measurement has become a surrogate for PFS and OS, regardless of cytogenetic abnormalities and has been reported as an important efficacy endpoint in clinical trials evaluating the role of HDT-ASCT [14, 15]. MRD status was not available for the majority of these patients, as routine measurement of MRD status was not an institutional practice. Nevertheless, our work is strengthened using data from a single center over a period of 10 years, reducing variability of practice patterns and improving reliability of our findings. More importantly, this data set incorporates collaboration of care between community physicians and an academic medical center for patients with NDMM and is a true reflection of the real-world management of these patients.
Conclusions
Cytogenetic abnormalities and lenalidomide-based induction and maintenance therapies had significant impact on efficacy outcomes after HDT-ASCT in a large cohort of patients with NDMM treated in a real-world setting. HRCAs were associated with inferior PFS but not OS when compared to standard-risk patients. Similarly, patients treated with lenalidomide-based maintenance regimens had superior PFS and OS when compared to non-lenalidomide-based therapies.
*P ≤ 0.05. NR: not reached; PFS: progression-free survival; OS: overall survival; CR: complete response; ORR: overall response rate, PI: proteasome inhibitor; VGPR: very good partial response.
| Supplementary Material | ▴Top |
Suppl 1. Patient, disease, and response characteristics for +1q standard-risk vs. +1q high-risk characteristics.
Suppl 2. Progression-free survival (a) and overall survival (b) based on +1q standard-risk vs. +1q high-risk cytogenetics.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
Hamza Hashmi is on advisory boards of Sanofi, BMS, and Janssen. No other authors have any conflict of interests to disclose.
Informed Consent
Since no human participants were present, there was no need to obtain an informed consent.
Author Contributions
AT, WW, and HH wrote the manuscript. All authors reviewed the final manuscript before submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Shridhar P, Chen Y, Khalil R, Plakseychuk A, Cho SK, Tillman B, Kumta PN, et al. A review of PMMA bone cement and intra-cardiac embolism. Materials (Basel). 2016;9(10):821.
doi pubmed pmc - Hariri O, Takayanagi A, Miulli DE, Siddiqi J, Vrionis F. Minimally invasive surgical techniques for management of painful metastatic and primary spinal tumors. Cureus. 2017;9(3):e1114.
doi pubmed pmc - Fadili Hassani S, Cormier E, Shotar E, Drir M, Spano JP, Morardet L, Collet JP, et al. Intracardiac cement embolism during percutaneous vertebroplasty: incidence, risk factors and clinical management. Eur Radiol. 2019;29(2):663-673.
doi pubmed - Pan HY, Wu TY, Chen HY, Wu JY, Liao MT. Intracardiac cement embolism: images and endovascular treatment. Circ Cardiovasc Imaging. 2021;14(4):e011849.
doi pubmed - Siddiqui AS, Goodarzi A, Majumdar T, Kaleekal T. A rare case of pulmonary cement embolism in a lung transplant patient. Respir Med Case Rep. 2018;24:63-64.
doi pubmed pmc - MacTaggart JN, Pipinos, II, Johanning JM, Lynch TG. Acrylic cement pulmonary embolus masquerading as an embolized central venous catheter fragment. J Vasc Surg. 2006;43(1):180-183.
doi pubmed - Bassawon R, Sirajuddin S, Martucci G, Shum-Tim D. Snare or scalpel: challenges of intracardiac cement embolism retrieval. Ann Thorac Surg. 2022;113(2):e107-e110.
doi pubmed - Anselmetti GC, Manca A, Montemurro F, Hirsch J, Chiara G, Grignani G, Carnevale Schianca F, et al. Percutaneous vertebroplasty in multiple myeloma: prospective long-term follow-up in 106 consecutive patients. Cardiovasc Intervent Radiol. 2012;35(1):139-145.
doi pubmed - Geraci G, Lo Iacono G, Lo Nigro C, Cannizzaro F, Cajozzo M, Modica G. Asymptomatic bone cement pulmonary embolism after vertebroplasty: case report and literature review. Case Rep Surg. 2013;2013:591432.
doi pubmed pmc - Rahimizadeh A, Hassani V, Soufiani H, Rahimizadeh A, Karimi M, Asgari N. Symptomatic pulmonary cement embolism after pedicle screw polymethylmethacrylate cement augmentation: A case report and review. Surg Neurol Int. 2020;11:18.
doi pubmed pmc - Waler AR, Sanchez KJ, Parikh AA, Okorie ON. A case of pulmonary cement embolism managed through symptomatic treatment. Case Rep Crit Care. 2020;2020:2425973.
doi pubmed pmc - Mansour A, Abdel-Razeq N, Abuali H, Makoseh M, Shaikh-Salem N, Abushalha K, Salah S. Cement pulmonary embolism as a complication of percutaneous vertebroplasty in cancer patients. Cancer Imaging. 2018;18(1):5.
doi pubmed pmc - Choe DH, Marom EM, Ahrar K, Truong MT, Madewell JE. Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty. AJR Am J Roentgenol. 2004;183(4):1097-1102.
doi pubmed - Patel Z, Sangani R, Lombard C. Cement pulmonary embolism after percutaneous kyphoplasty: An unusual culprit for non-thrombotic pulmonary embolism. Radiol Case Rep. 2021;16(11):3520-3525.
doi pubmed pmc - Debbie Jiang MD, Alfred Ian Lee MD. Thrombotic risk from chemotherapy and other cancer therapies. Cancer Treat Res. 2019;179:87-101.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


