| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 13, Number 3, June 2024, pages 71-78
Predictors of Non-Variceal Hemorrhage in a National Cohort of Patients With Chronic Liver Disease
Amber Afzala, e, f , Preethi Kesavanb, e, Luo Suhongc, Brian F. Gageb, Kevin Korenblatd, Martin Schoenc, Kristen Sanfilippoa, c
aDivision of Hematology, Department of Medicine, Washington University in St Louis, MO, USA
bDepartment of Medicine, Washington University in St. Louis, MO, USA
cDivision of Oncology, Department of Medicine, John Cochran VA Medical Center, St. Louis, MO, USA
dDivision of Gastroenterology, Department of Medicine, Washington University in St. Louis, MO, USA
eThese authors contributed equally to the study.
fCorresponding Author: Amber Afzal, Division of Hematology, Department of Medicine, Washington University in St. Louis, MO, USA
Manuscript submitted January 25, 2024, accepted May 4, 2024, published online June 28, 2024
Short title: Predictors of Non-Variceal Hemorrhage in CLD
doi: https://doi.org/10.14740/jh1214
| Abstract | ▴Top |
Background: Non-variceal hemorrhage in patients with chronic liver disease (CLD) increases morbidity, mortality, and healthcare costs. There are limited data on risk factors for non-variceal hemorrhage in the CLD population. The aim of this study was to assess the predictive value of various clinical and laboratory parameters for non-variceal hemorrhage in CLD patients.
Methods: We conducted a retrospective cohort study of US veterans diagnosed with CLD between 2002 and 2018 within the Veterans Health Administration database. We derived candidate variables from existing risk prediction models for hemorrhage, risk calculators for severity of liver disease, Charlson index of prognostic comorbidities, and prior literature. We used a competing risk analysis to study the relationship between putative risk factors and incidence of non-variceal hemorrhage in patients with CLD.
Results: Of 15,183 CLD patients with no history of cancer or anticoagulation use, 674 experienced non-variceal hemorrhage within 1 year of CLD diagnosis. In multivariable analysis, 11 of the 26 candidate variables independently predicted non-variceal hemorrhage: race, international normalized ratio (INR) > 1.5, bilirubin ≥ 2 mg/dL, albumin ≤ 3.5 g/dL, anemia, alcohol abuse, antiplatelet therapy, chronic kidney disease, dementia, proton pump inhibitor prescription, and recent infection.
Conclusions: In this study of almost 15,000 veterans, risk factors for non-variceal bleeding within the first year after diagnosis of CLD included non-Caucasian race, laboratory parameters indicating severe liver disease and recent infection in addition to the risk factors for bleeding observed in a general non-CLD population.
Keywords: Hemorrhage; Risk factors; Chronic liver disease; Cirrhosis
| Introduction | ▴Top |
Hemorrhage is a frequent complication of chronic liver disease (CLD) [1]. Among the 4.5 million Americans with CLD [2], hemorrhage frequently leads to morbidity, mortality and increased health-care costs [3, 4]. Hemorrhage in patients with CLD is classified as variceal hemorrhage (i.e., originating from gastroesophageal varices) [1], and non-variceal hemorrhage (e.g., epistaxis, procedure-related [5], non-variceal gastrointestinal, and intracranial hemorrhage [6]). Variceal hemorrhage is a consequence of portal hypertension [7], and studies have reported risk factors for variceal hemorrhage [8-10]. There are, however, sparse data on risk factors of non-variceal hemorrhage in patients with CLD.
Non-variceal hemorrhage can trigger decompensation of liver disease, spontaneous bacterial peritonitis, hepatorenal syndrome and hepatic encephalopathy [11]. Although patients with CLD experience non-variceal hemorrhage less frequently than variceal hemorrhage [1], there is no difference between mortality, length of hospital stay and readmission rates between patients presenting with variceal and non-variceal hemorrhage [12]. A better understanding of the risk factors for non-variceal hemorrhage can guide preventive strategies against hemorrhage in patients with CLD, and thus improve clinical outcomes.
Patients with CLD have a unique hemostatic profile [13], and hence, traditional risk factors for hemorrhage, such as low platelet count and increasing international normalized ratio (INR) values [14-17], may not predict hemorrhage. Preclinical studies show that thrombocytopenia in CLD patients is compensated for by elevations in von Willebrand Factor (vWF) activity [18], and elevated INR is balanced by a decrease in certain anticoagulant factors (e.g., antithrombin, protein C and protein S), and increases in factor VIII levels [19]. There is, hence, a need to study the value of these laboratory parameters in predicting hemorrhage in the CLD population.
In this study, we used a national cohort of veterans with CLD to identify the clinical and laboratory parameters that predict non-variceal hemorrhage.
| Materials and Methods | ▴Top |
The St. Louis Veterans Affairs Medical Center (VAMC) and Washington University School of Medicine Institutional Review Boards (IRB) approved this study. The study was performed in accordance with the Declaration of Helsinki. This study analyzed data retrospectively using de-identified electronic medical records, so an informed consent was not obtained (in accordance with the VAMC and the Washington University in St Louis IRB policy).
Data source
Using the national Veterans Health Administration (VHA) data, we conducted a retrospective single-cohort study. Information on demographics, comorbidities, outcomes, vital signs, laboratory values and prescription data for medication (antiplatelets, anticoagulants, proton pump inhibitors) were obtained from the Veterans Informatics and Computing Infrastructure (VINCI). Date and cause of death were obtained from the Veterans Administration Vitals Statistics [20].
Study population
We included patients 18 years or older diagnosed with CLD between October 1, 2002 and September 30, 2016, and followed them until 2018 (Fig. 1). We used International Classification of Diseases (ICD)-9/10 codes to identify CLD. To improve diagnostic accuracy, we used a previously validated algorithm [21] where patients are required to have two ICD-9 or -10 codes to qualify for CLD diagnosis: one for CLD (i.e., hepatitis or cirrhosis) and one for a common clinical presentation associated with CLD (e.g., hepatic encephalopathy, hepatorenal syndrome, etc.) (Supplementary Material 1, www.thejh.org).
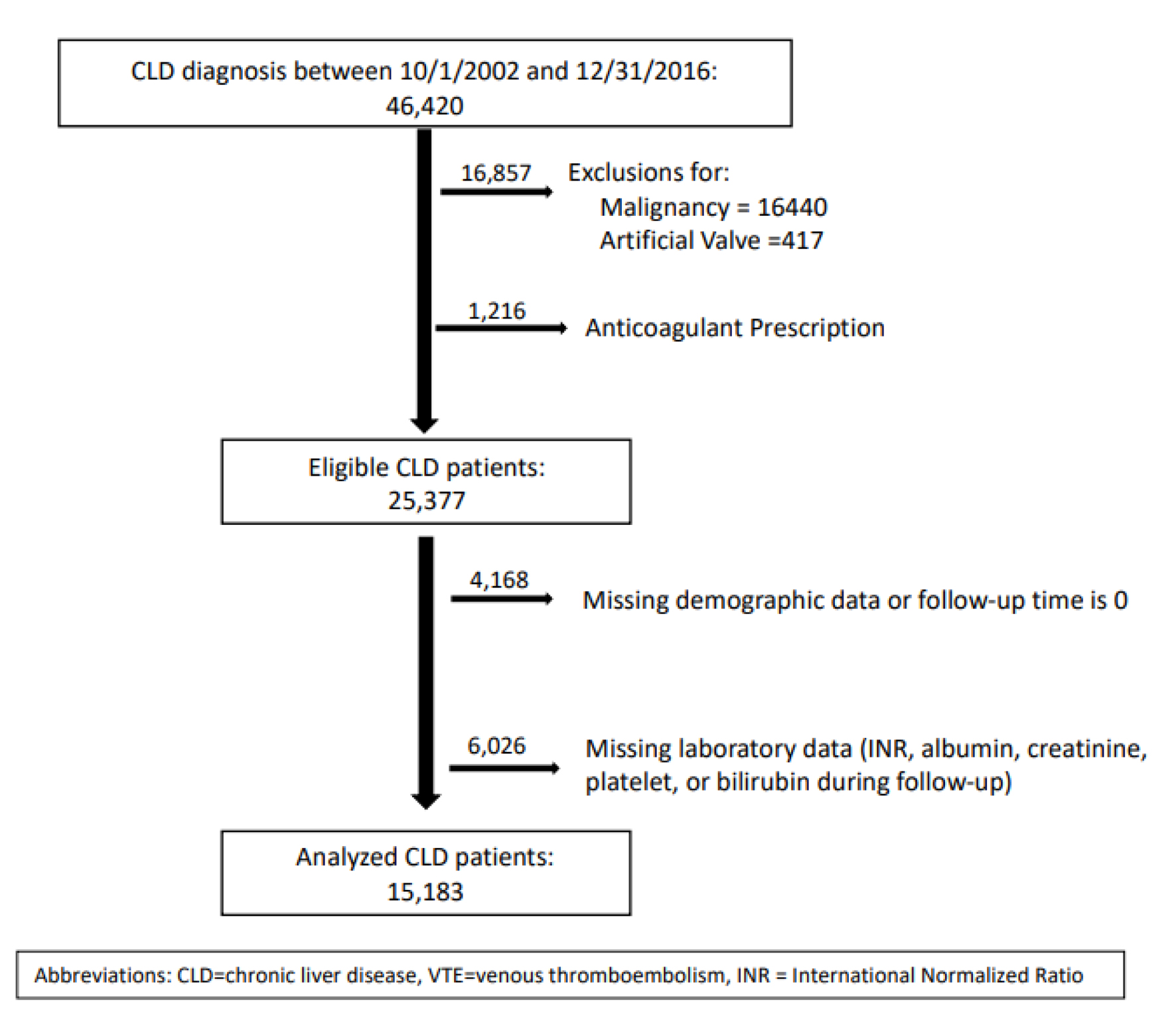 Click for large image | Figure 1. CONSORT diagram. CLD: chronic liver disease; VTE: venous thromboembolic; INR: international normalized ratio. |
To exclude patients who could be receiving anticoagulant therapy, we excluded those with a history of artificial heart valve and those with an active prescription for anticoagulant therapy during study period. We also excluded patients with history of malignancy within 5 years prior to CLD diagnosis as these patients have additional risks for bleeding. We followed patients up to the date of hemorrhage, loss to follow-up, or 12 months after CLD diagnosis, whichever came first.
Detection of outcomes
The primary outcome was occurrence of a non-variceal hemorrhage within 12 months of CLD diagnosis. A non-variceal hemorrhage was identified using previously validated ICD-9/10 codes in 1) any position for gastrointestinal bleeding, intracranial bleeding, epistaxis and gross hematuria, and 2) in the primary position for hemorrhage at all other sites [22-25] (Supplementary Material 2, www.thejh.org). We excluded ICD codes for non-specific gastrointestinal bleeding, e.g., melena and hematemesis that likely denote variceal hemorrhage in patients with CLD.
Selection of candidate variables
We studied the role of 26 clinical and laboratory parameters in predicting hemorrhage among patients with liver disease. We derived candidate variables from demographic characteristics (age, gender, race), existing risk-prediction models for hemorrhage [14-16, 26], risk-prediction models for severity of liver disease (i.e., Child-Pugh [27] and MELD [28] scores), Charlson index for prognostic comorbidities [29], and prior literature [30]. Demographic variables were studied as fixed variables and values closest to the date of CLD diagnosis were used for analyses. Comorbidities were identified within 12 months prior to CLD diagnosis and used as fixed variables. Laboratory values (e.g., INR) and prescriptions (e.g., antiplatelet therapy) were used as time-varying variables to account for their temporal association with hemorrhage (Supplementary Material 3, www.thejh.org).
Statistical analysis
We analyzed the association between candidate variables and hemorrhage in a time-to-event analysis: time 0 was the date of CLD diagnosis and the outcome of interest was first non-variceal hemorrhage during follow-up. We used competing risk model by the methods of Fine and Gray to perform a multivariable analysis while accounting for the competing risk of non-hemorrhage related death [31]. A P-value of < 0.05 was considered significant. All statistical analyses were performed using SAS version 9.2.
| Results | ▴Top |
Most patients were Caucasian males with a median age of 58 years. Of the 15,183 patients with CLD who were included in the study (Fig. 1), 674 (4.4%) experienced non-variceal hemorrhages within 12 months of CLD diagnosis. Patients who experienced hemorrhage were more likely to be younger, non-Caucasian, with a history of congestive cardiac failure, anemia, alcohol abuse, antiplatelet therapy, and recent infection. They were also more likely to have laboratory abnormalities of INR, bilirubin, albumin, and creatinine clearance and to have higher MELD scores (14 versus 12.8, P = 0.0004) (Table 1). Of the 674 hemorrhages observed during follow-up, most occurred within the upper gastrointestinal tract followed by the lower gastrointestinal tract, intracranial, and nasal/respiratory sites (Table 2).
 Click to view | Table 1. Demographic and Clinical Characteristics of Patients With Chronic Liver Disease |
 Click to view | Table 2. Site of Hemorrhage |
In the multivariable analysis, 11 of the 26 candidate variables were found to be independent risk factors for non-variceal hemorrhage: non-Caucasian race, abnormal INR, high bilirubin, low albumin, anemia, alcohol abuse, antiplatelet therapy, chronic kidney disease, dementia, recent infection, and use of proton pump inhibitors. Among these risk factors, low serum albumin, chronic kidney disease, dementia and infections predicted the highest risk of hemorrhage (Table 3).
 Click to view | Table 3. Multivariable Analysis of Risk Factors for Non-Variceal Hemorrhage |
| Discussion | ▴Top |
In a nationwide cohort of 15,183 veterans with CLD, we found a 4.4% annual incidence of non-variceal hemorrhage, and we identified clinical and laboratory predictors of non-variceal hemorrhage in this population.
We analyzed risk of hemorrhage associated with laboratory parameters that predict severity of liver disease in the MELD [28] and Child-Pugh classifications [27]. We chose the cut-offs of these laboratory parameters (bilirubin, albumin, and creatinine clearance) based on the definitions used in the MELD and Child-Pugh classifications. We chose the cut-off for INR based on recent literature showing uniform elevation in risk of hemorrhage at all INR values above 1.5 [17]. All these laboratory abnormalities predicted higher risk of hemorrhage suggesting that the severity of liver disease at diagnosis correlates with the risk of non-variceal hemorrhagic complications in the following year. While low albumin represents severe liver disease, this could also affect the risk of hemorrhage by creating an inflammatory milieu [32, 33]. The association between hypoalbuminemia and hemorrhage should be explored further in future studies. Ascites and hepatic encephalopathy, although components of Child-Pugh classification, did not predict hemorrhage in this study. The lack of association between ascites and hemorrhage could be due to non-specific coding of ascites, which may or may not be a consequence of CLD, as suggested by other administrative database studies [21]. Hepatic encephalopathy was coded in less than 1% of our study cohort, which made it hard to find a putative association with hemorrhage. Our analysis however, found a 2.5-fold increase in the risk of hemorrhage in people with “dementia”, which could represent episodes of hepatic encephalopathy in patients with CLD.
The results from this study support prior preclinical research addressing hemostasis in patients with CLD. For example, patients with CLD did not experience a higher risk of hemorrhage with thrombocytopenia. This lack of association supports the preclinical studies demonstrating rebalancing of hemostasis in CLD patients with thrombocytopenia by elevated vWF levels [18, 34]. We found 2.5-fold risk of hemorrhage with recent infection in this analysis, which supports the preclinical finding that infections can promote hemorrhage in CLD by producing heparin like effect, detectable on heparinase-modified thromboelastography [30, 35].
Some of the risk factors for hemorrhage in this cohort of CLD patients concur with the risk factors for hemorrhage observed in a general non-CLD population, e.g., anemia, alcohol abuse, and antiplatelet therapy [14-16]. Dementia predicted a higher risk of hemorrhage likely due to compromised self-care and an increased risk of falls [36]. Other risk factors for hemorrhage in a general non-CLD population, e.g., age and hypertension [14-16] did not predict hemorrhage in CLD patients. The lack of association between age and hemorrhage could be due to survivorship bias where patients with severe liver disease do not live long enough [37] to experience hemorrhage. The restricted age range of our study population with few veterans older than 75 years also makes it harder to find this association. Patients with severe liver disease are more likely to have hypotension than hypertension [38], and this may explain the lack of association between hypertension and hemorrhage in this cohort. Moreover, we identified non-Caucasian race to be at a higher risk of non-variceal hemorrhage, which represents a novel finding. This could result from disparities in socioeconomic status, access to healthcare, cultural practices or diverse genetic background and deserves further investigation.
The most frequent site of non-variceal hemorrhage in patients with CLD was upper gastrointestinal tract (60%). This was followed by lower gastrointestinal tract (19.3%) and intracranial (9.9%) sites (Table 2). Patients with liver disease are pre-disposed to vascular ectasias [11] and peptic ulcer disease [39], which could explain the higher risk of upper gastrointestinal hemorrhage. Another plausible explanation for higher risk of gastrointestinal hemorrhage can be stress-related mucosal ulceration, which can be triggered by events like infections. The frequency of spontaneous non-variceal hemorrhage at sites other than the gastrointestinal and intracranial location was low (< 1% per year) in this cohort.
We utilized the VHA database to conduct this study as it provides access to patient charts, vital signs, laboratory parameters and pharmacy data from both inpatient and outpatients encounters. This is helpful for an unbiased determination of risk factors for hemorrhage as outcomes associated with parameters observed during hospitalization alone could be confounded by the reason for hospitalization and interventions performed during hospitalization, e.g., transfusions. Moreover, we assessed vital signs, laboratory parameters and medication as time-dependent variables and utilized all values during the study period to determine temporal association of these variables with hemorrhage. We used previously validated methods to identify study population and outcomes [21-25].
This study has several limitations. We relied on ICD-9 and -10 codes for identification of hemorrhage, so inclusion of events was contingent on accuracy of coding. The coding provider may have misclassified variceal hemorrhage as “non-variceal”. We did not have access to blood transfusion data and hence, severity of hemorrhage could not be accurately described. Moreover, we were unable to capture any events outside of the VHA system. VHA database underrepresents females, and thus results should be confirmed in a cohort more inclusive of females. While retrospective analysis establishes association, it is hard to elucidate causation. For example, anemia and prescription for proton pump inhibitors likely represent consequences of hemorrhage rather than “causes” for hemorrhage. Despite these limitations, this study provides a comprehensive assessment of the clinical and laboratory predictors of non-variceal hemorrhage in a national cohort of CLD patients. This study has the potential to guide physicians in risk-stratifying patients for hemorrhage at the time of CLD diagnosis and encourage them to perform a careful assessment of the risk versus benefit of certain interventions like antiplatelet therapy that predispose to hemorrhage. This study will also generate rationale for future research for example, development of a risk-prediction model for hemorrhage specific to patients with CLD.
In summary, non-Caucasian race, severity of liver disease, anemia, alcohol abuse, antiplatelet therapy, chronic kidney disease, dementia, infections, and prescriptions for proton pump inhibitors are associated with a higher risk of non-variceal hemorrhage in the first year after CLD diagnosis. Knowledge of risk factors for hemorrhage in CLD can inform management of this patient population (e.g., avoidance of antiplatelet therapy without a clear indication) and potentially reduce mortality and healthcare costs associated with non-variceal bleeds in this population [12].
| Supplementary Material | ▴Top |
Suppl 1. Identification of chronic liver disease using ICD-9/10 codes.
Suppl 2. Identification of hemorrhage using ICD-9/10 codes.
Suppl 3. Definition and Measurement of Covariates.
Acknowledgments
The contents do not represent the views of the US Department of Veterans Affairs or the United States Government. This material was produced using resources and facilities at the Saint Louis Veterans Affairs Medical Center, Saint Louis, Missouri.
Financial Disclosure
This work was supported by the National Heart, Lung, and Blood Institute (1K01HL136893-01, 5K12 HL087107-95, and the NIH Loan Repayment Program to Dr Sanfilippo) and the National Center for Advancing Translational Sciences grant (UL1 TR000448).
Conflict of Interest
None to declare.
Informed Consent
Not applicable (this publication analyzed de-identified electronic medical records with no identifiable image or information in the final publication).
Author Contributions
Conceptualization and study design: AA, PK, KMS. Data acquisition: AA, LS, KMS. Data analysis and interpretation: AA, PK, LS, BFG, KK, MS, KMS. Manuscript preparation: AA, PK, LS. Manuscript review: AA, PK, LS, KK, BFG, MS, KMS.
Data Availability
Datasets used and analyzed during the current study are available in the Veterans Medical Affairs password protected electronic system. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Sharara AI, Rockey DC. Gastroesophageal variceal hemorrhage. N Engl J Med. 2001;345(9):669-681.
doi pubmed - CDC: Liver Disease. 2020. Available from: https://www.cdc.gov/nchs/fastats/liver-disease.htm.
- Desai AP, Mohan P, Nokes B, Sheth D, Knapp S, Boustani M, Chalasani N, et al. Increasing economic burden in hospitalized patients with cirrhosis: analysis of a national database. Clin Transl Gastroenterol. 2019;10(7):e00062.
doi pubmed pmc - Adam V, Barkun AN. Estimates of costs of hospital stay for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health. 2008;11(1):1-3.
doi pubmed - Schepis F, Turco L, Bianchini M, Villa E. Prevention and management of bleeding risk related to invasive procedures in cirrhosis. Semin Liver Dis. 2018;38(3):215-229.
doi pubmed - Hoya K, Tanaka Y, Uchida T, Takano I, Nagaishi M, Kowata K, Hyodo A. Intracerebral hemorrhage in patients with chronic liver disease. Neurol Med Chir (Tokyo). 2012;52(4):181-185.
doi pubmed - Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362(9):823-832.
doi pubmed - Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, Kobayashi M. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981;27(4):213-218.
doi pubmed - Ma JL, He LL, Jiang Y, Yang JR, Li P, Zang Y, Wei HS. New model predicting gastroesophageal varices and variceal hemorrhage in patients with chronic liver disease. Ann Hepatol. 2020;19(3):287-294.
doi pubmed - North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319(15):983-989.
doi pubmed - Kalafateli M, Triantos CK, Nikolopoulou V, Burroughs A. Non-variceal gastrointestinal bleeding in patients with liver cirrhosis: a review. Dig Dis Sci. 2012;57(11):2743-2754.
doi pubmed - Tandon P, Bishay K, Fisher S, Yelle D, Carrigan I, Wooller K, Kelly E. Comparison of clinical outcomes between variceal and non-variceal gastrointestinal bleeding in patients with cirrhosis. J Gastroenterol Hepatol. 2018;33(10):1773-1779.
doi pubmed - Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147-156.
doi pubmed - Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151(3):713-719.
doi pubmed - Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-1100.
doi pubmed - Klok FA, Hosel V, Clemens A, Yollo WD, Tilke C, Schulman S, Lankeit M, et al. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur Respir J. 2016;48(5):1369-1376.
doi pubmed - Afzal A, Gage BF, Suhong L, Schoen MW, Korenblat K, Sanfilippo KM. Different risks of hemorrhage in patients with elevated international normalized ratio from chronic liver disease versus warfarin therapy, a population-based retrospective cohort study. J Thromb Haemost. 2022;20(7):1610-1617.
doi pubmed pmc - Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG, Leebeek FW. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44(1):53-61.
doi pubmed - Tripodi A, Primignani M, Chantarangkul V, Dell'Era A, Clerici M, de Franchis R, Colombo M, et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137(6):2105-2111.
doi pubmed - VA Vital Statistics. 2022.
- Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47(5):e50-e54.
doi pubmed pmc - Shehab N, Ziemba R, Campbell KN, Geller AI, Moro RN, Gage BF, Budnitz DS, et al. Assessment of ICD-10-CM code assignment validity for case finding of outpatient anticoagulant-related bleeding among Medicare beneficiaries. Pharmacoepidemiol Drug Saf. 2019;28(7):951-964.
doi pubmed - Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118(2):253-262.
doi pubmed - Delate T, Jones AE, Clark NP, Witt DM. Assessment of the coding accuracy of warfarin-related bleeding events. Thromb Res. 2017;159:86-90.
doi pubmed - Joos C, Lawrence K, Jones AE, Johnson SA, Witt DM. Accuracy of ICD-10 codes for identifying hospitalizations for acute anticoagulation therapy-related bleeding events. Thromb Res. 2019;181:71-76.
doi pubmed - Decousus H, Tapson VF, Bergmann JF, Chong BH, Froehlich JB, Kakkar AK, Merli GJ, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79.
doi pubmed - Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85.
pubmed - Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91-96.
doi pubmed - Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
doi pubmed - Montalto P, Vlachogiannakos J, Cox DJ, Pastacaldi S, Patch D, Burroughs AK. Bacterial infection in cirrhosis impairs coagulation by a heparin effect: a prospective study. J Hepatol. 2002;37(4):463-470.
doi pubmed - Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509.
- Che R, Huang X, Zhao W, Jiang F, Wu L, Zhang Z, Bian T, et al. Low serum albumin level as a predictor of hemorrhage transformation after intravenous thrombolysis in ischemic stroke patients. Sci Rep. 2017;7(1):7776.
doi pubmed pmc - Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181-193.
doi pubmed pmc - Tripodi A, Primignani M, Chantarangkul V, Clerici M, Dell'Era A, Fabris F, Salerno F, et al. Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology. 2006;44(2):440-445.
doi pubmed - Kuo MT, Yang SC, Lu LS, Hsu CN, Kuo YH, Kuo CH, Liang CM, et al. Predicting risk factors for rebleeding, infections, mortality following peptic ulcer bleeding in patients with cirrhosis and the impact of antibiotics prophylaxis at different clinical stages of the disease. BMC Gastroenterol. 2015;15:61.
doi pubmed pmc - Shaw FE. Falls in cognitive impairment and dementia. Clin Geriatr Med. 2002;18(2):159-173.
doi pubmed - D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217-231.
doi pubmed - Vora RS, Subramanian RM. Hypotension in cirrhosis. Clin Liver Dis (Hoboken). 2019;13(6):149-153.
doi pubmed pmc - Gonzalez-Gonzalez JA, Garcia-Compean D, Vazquez-Elizondo G, Garza-Galindo A, Jaquez-Quintana JO, Maldonado-Garza H. Nonvariceal upper gastrointestinal bleeding in patients with liver cirrhosis. Clinical features, outcomes and predictors of in-hospital mortality. A prospective study. Ann Hepatol. 2011;10(3):287-295.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


