| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 13, Number 1-2, April 2024, pages 39-45
Middle Meningeal Artery Embolization in Acute Leukemia Patients Presenting With Subdural Hematoma
Binoy Yohannana, Juan Carlos Martinez Gutierrezb, Peng Roc Chenb, Adan Riosa, c
aDivision of Hematology/Oncology, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX 77030, USA
bDivision of Neurosurgery, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX 77030, USA
cCorresponding Author: Adan Rios, Division of Hematology/Oncology, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX 77030, USA
Manuscript submitted November 11, 2023, accepted December 30, 2023, published online January 10, 2024
Short title: MMA Embolization in Acute Leukemia
doi: https://doi.org/10.14740/jh1215
| Abstract | ▴Top |
Intracerebral hemorrhage is a potentially fatal complication in patients with acute leukemia and contributing factors include thrombocytopenia and coagulopathy. Patients with acute leukemia may develop subdural hematoma (SDH) spontaneously or secondary to trauma. In patients with acute leukemia and SDH, the surgical evacuation of the hematoma causes significant morbidity and mortality. New approaches and strategies to reduce the need for surgical evacuation are needed to improve outcomes in patients with acute leukemia and intracerebral hemorrhage. We report two cases of acute SDH in patients with acute leukemia successfully treated with middle meningeal artery embolization, a minimally invasive interventional radiology technique, obviating the need for a surgical intervention. The first patient with acute promyelocytic leukemia (APL) presented with coagulopathy and developed an acute SDH after a fall. The second patient with acute myeloid leukemia presented with gum bleeding and also sustained an acute SDH after a fall. Both patients underwent middle meningeal artery embolization for treating their SDHs while actively receiving induction chemotherapy for acute leukemia. Both patients had resolution of their acute SDH and are in remission from their acute leukemia. Middle meningeal artery embolization is a very effective, and within the context of this setting, a novel, minimally invasive technique for management of SDH in acute leukemia patients, which can prevent the need for surgical interventions with its associated comorbidities and high risk of fatal outcomes in patients with acute leukemia and acute SDH.
Keywords: Acute leukemia; Subdural hematoma; Middle meningeal artery embolization
| Introduction | ▴Top |
Due to inherent abnormalities of the coagulation system, patients with acute leukemia have a higher propensity for spontaneous intracranial hemorrhage (ICH), a leading cause of morbidity and mortality. Dayyani et al reported a single-center retrospective review of ICH with its incidence among acute leukemia patients of 4.8%, the highest incidence seen in chronic myeloid leukemia (CML) patients in blast crisis [1]. Disseminated intravascular coagulation (DIC) with ICH is a leading cause of early deaths in acute promyelocytic leukemia (APL). In addition to general vascular risk factors, other contributing factors of ICH in acute leukemia patients include thrombocytopenia, hyperleukocytosis, and leukemic infiltration [2-4]. The duration of severe thrombocytopenia (< 10 × 109/L) correlates with major bleeding events [5, 6]. In patients with hematological malignancies and ICH, prophylactic platelet transfusions to keep the platelet count above 10 × 109/L have been proven to be effective in lowering bleeding events [7].
Surgical intervention in patients with acute leukemia can be quite challenging and is a morbid procedure in the setting of severe thrombocytopenia and coagulopathy. In patients with acute leukemia and spontaneous or secondary subdural hematoma (SDH), surgical treatment with a single burr hole or even craniotomy may be required to avoid the impact of a significant mass effect. Chemotherapy for acute leukemia further complicates this clinical scenario with its associated myelosuppression. There is an unmet need for a minimally invasive intervention to manage all types of acute ICH in patients with acute leukemia. We report two cases of SDH in patients with acute leukemia successfully managed with middle meningeal artery (MMA) embolization at our institution.
| Case Reports | ▴Top |
Case 1
A patient in her 50s with a history of spina bifida, diabetes, and hypertension, presented to an outside institution emergency room (ER) with headache after a fall. There was no history of seizures or loss of consciousness. In the outside ER, she had a computed tomography (CT) of the head that showed a small SDH, 1.0 cm in diameter size with 6-mm midline shift. The patient was discharged home with instructions to return if the symptoms worsened. A few days later, the patient had worsening headaches, blurry vision, nausea, and vomiting. Repeated imaging showed a worsening left SDH measuring now 1.2 cm in thickness with a 6-mm midline shift (Fig. 1a). She was neurologically unstable and was life-flighted to our hospital for a higher level of care. Upon arrival at our institution the patient endorsed having had easy bruising, gum bleeding, fatigue, bleeding over her torso and extremities for 3 weeks prior to admission. Blood-tinged urine was also noted during clean intermittent catheterizations due to her neurogenic bladder. She denied any fevers, chills, night sweats, melena, or hematochezia. On examination, the patient was alert, awake and oriented to time, place, and person and was able to follow commands. Pupils were 4 mm bilaterally and reactive. Cranial nerves were intact. Motor strength was preserved. The patient had previously undergone a lower extremity below-knee amputation for chronic osteomyelitis which was well healed. The skin examination revealed diffuse petechial rash. Laboratory values at presentation included a white blood cell (WBC) count of 23 × 109/L, platelets 13 × 109/L, hemoglobin (Hb) 9.5 g/dL, fibrinogen 157 mg/dL, prothrombin time (PT) 15.7 s, and partial thromboplastin time (PTT) 27.6 s. A pathologist review of the admission peripheral blood smear revealed 20% blasts with the presence of Auer rods, and a diagnosis of APL was clinically suspected. The patient received a platelet transfusion and 10 units of cryoprecipitate. Given her obvious APL-related coagulopathy, all-trans retinoic acid (ATRA) was started immediately, and 12 mg of intravenous idarubicin was given for elevated WBC count. A fluorescence in situ hybridisation (FISH) result from the peripheral blood showed t(15:17), confirming the diagnosis of APL. Bone marrow biopsy showed hypercellular marrow with 85% aberrant promyelocytes by differential count. FISH studies from the bone marrow also showed PML-RARA translocation. Definitive therapy for high-risk APL was started with ATRA plus arsenic trioxide. On day 5, after neurological stabilization, diagnosis, and initiation of therapy for her acute leukemia, a cerebral angiogram with embolization of the left MMA was successfully done to prevent any further deterioration of her neurological status. The details of the procedure are given below.
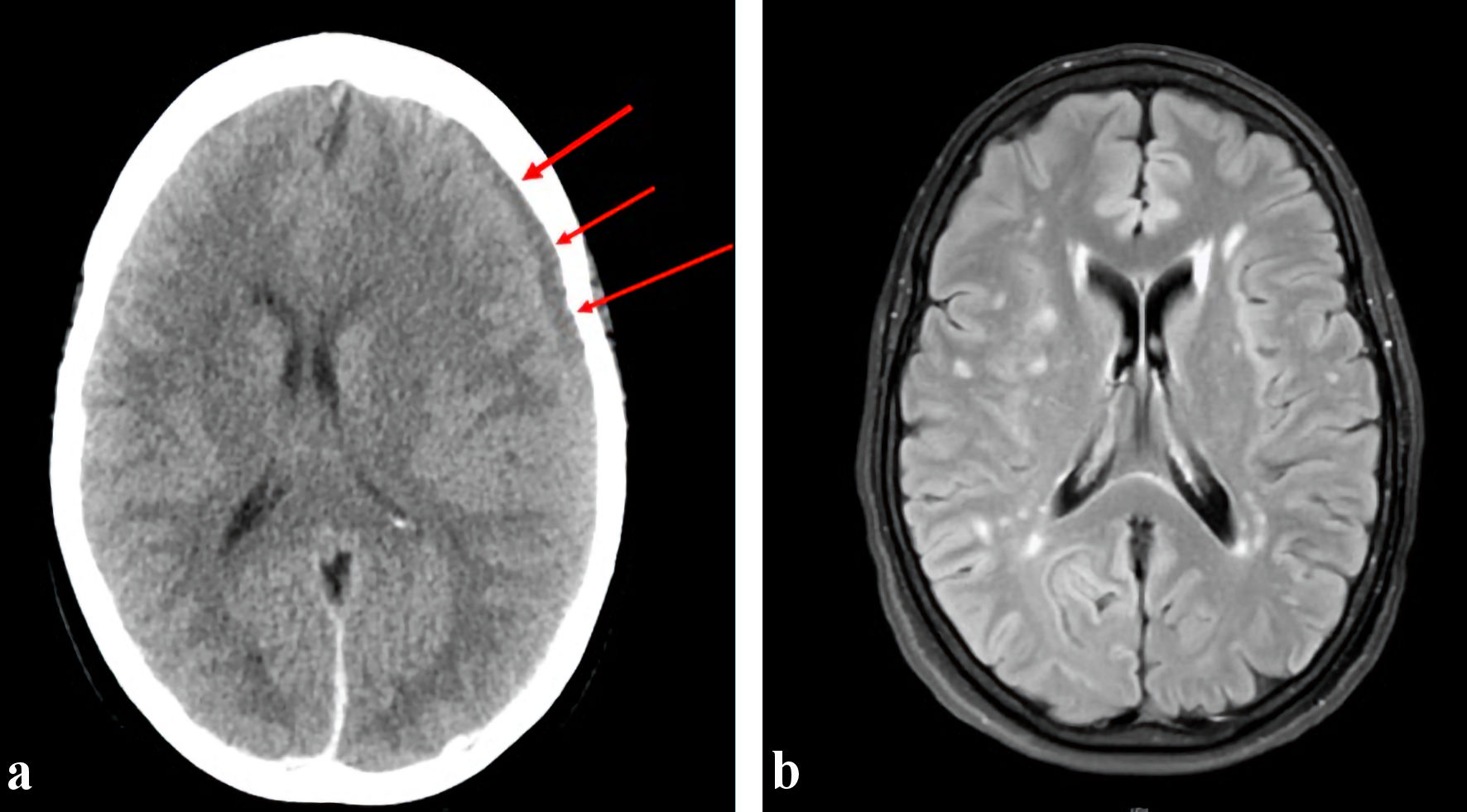 Click for large image | Figure 1. (a) Preoperative CT head showing left cerebral convexity subdural hematoma (arrows), measuring up to 1.1 cm in thickness. There is an associated rightward midline shift of 6 mm at the level of the foramen of Monro and trace subarachnoid hemorrhage in the left frontal lobe. (b) MRI brain done 7 months later showing resolved subarachnoid hemorrhage along the left cerebral convexity and right parafalcine region with no evidence of residual or recurrence hemorrhage. CT: computed tomography; MRI: magnetic resonance imaging. |
Informed consent was obtained after carefully reviewing the potential risks and benefits with the patient. The patient was intubated by the anesthesia team. The left femoral artery was accessed using a single-wall micropuncture technique, and a 6-French sheath was placed. Intraarterial infusion of nitroglycerin 200 mg was done to prevent vasospasm. A 6-French Envoy MPC catheter was coaxially advanced over a 5-French penumbra select catheter over a 0.035 Terumo glidewire through the sheath into the aortic arch. Using the roadmap projection, a Headway Duo microcatheter over a Traxcess 14 microwire was advanced via the 6-French Envoy MPC to access the left internal maxillary artery and, subsequently, the left MMA. The microwire was removed and an angiogram with runoff was performed to confirm placement. Gentle infusion of Onyx 18 embolization material was then initiated. A repeat angiography revealed complete occlusion of the left MMA (Fig. 2a, b). The patient tolerated the procedure well and was neurologically intact. A repeated bone marrow biopsy done after the completion of induction therapy showed a complete hematological remission. The patient has completed all her scheduled consolidation therapies and remains in complete molecular remission. A repeated brain magnetic resonance imaging (MRI) done 7 months later showed complete resolution of the SDH (Fig. 1b).
 Click for large image | Figure 2. Cerebral angiogram showing the left middle meningeal artery (MMA) region (a). Post-embolization images (b) show a completely occluded left MMA (arrows). |
Case 2
A patient in his 40s with no significant past medical history presented with a 1-month history of bleeding gums, generalized weakness, 60-pound weight loss, and a history of two recent falls. The last fall was 1 week prior to admission. The patient had a syncopal episode and sustained head trauma. The patient reported subjective fevers, chills, night sweats, blurry vision, headaches, and nausea without vomiting. There was no history of hemoptysis, hematemesis, hematuria, or hematochezia. The patient reported smoking cigarettes occasionally and being a social drinker but denied history of intravenous drug use and had no family history of cancer or leukemia. On examination, the patient was alert and oriented to time, place, and person and had pallor but no icterus. Gum hypertrophy and bleeding was noted. Cranial nerves were intact, and there was no focal neurological deficit. Initial laboratory tests results were notable for a WBC of 45 × 109/L, Hb of 5.4 g/dL, and platelet count of 11 × 109/L. Peripheral blood smear showed 60% blasts and Auer rods. A CT of the head showed bilateral acute on chronic SDHs measuring up to approximately 2.0 cm on the left and 1.3 cm on the right with stable 3-mm left-to-right midline shift (Fig. 3a). The patient underwent embolization of the bilateral MMA and tolerated the procedure well. The details of the procedure are given below.
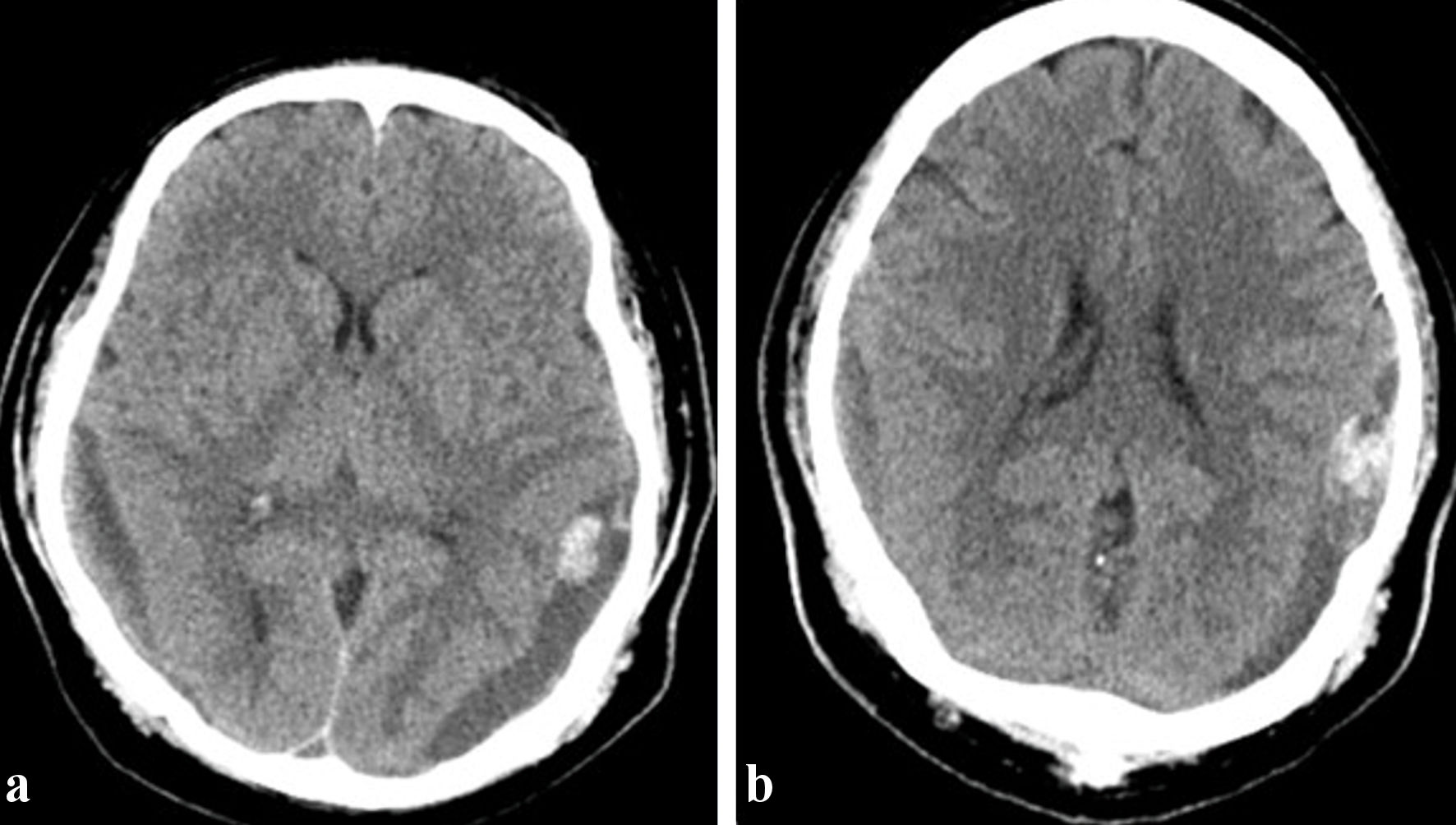 Click for large image | Figure 3. Subdural collections overlying the right and left parieto-occipital convexities and measuring up to 2.0 cm on the left and 1.3 cm on the right, with a mass effect of 3 mm mild left-to-right midline shift (a). (b) Interval improvement of the mass effect on the left with persistent 1 mm midline shift toward the right. |
The right femoral artery was accessed using a single-wall micropuncture technique, and a 5-French sheath was placed. A 6-French Vert catheter was coaxially advanced over a 5-French penumbra select catheter over a 0.035 Terumo glidewire through the sheath into the aortic arch to select the involved arteries. Then, 5,000 units of heparin were administered. Using the roadmap projection, an SL 10 straight-tip microcatheter over a Synchro soft O14 microwire was advanced via the 6-French Envoy MPC to access the right internal maxillary artery and, subsequently, the right MMA. The microwire was removed, and angiography was performed to confirm placement. A gentle infusion of Onyx 18 was then initiated and continued until there was evidence of distal stasis. A repeat angiography revealed significantly diminished filling of this branch. Successive coils were then deployed. Using the second roadmap projection, an SL 10 straight-tip microcatheter over a Synchro soft O14 microwire was advanced via the 6-French Envoy MPC to access the left internal maxillary artery and, subsequently, the left MMA. The microwire was removed, and angiography was performed to confirm placement. A gentle infusion of Onyx 18 was then initiated and continued until there was evidence of distal stasis. A repeat angiography revealed significantly diminished filling of this branch. Successive coils were then deployed.
A post-embolization CT of the head showed interval improvement of the mass effect on the left with a persistent 1-mm midline shift toward the right (Fig. 3b). Bone marrow aspiration and biopsy confirmed acute myeloid leukemia with 31% blasts. The patient received induction chemotherapy with cladribine, subcutaneous cytarabine, and venetoclax. The hospital course was complicated by neutropenic fever and Streptococcus pneumoniae bacteremia, which was treated with appropriate antibiotics. A repeat bone marrow biopsy on day 21 showed a complete remission, and the patient was then referred to an outside institution for continuation of medical care. There was no evidence of recurrent SDH.
| Discussion | ▴Top |
Acute SDHs are more often seen in the setting of trauma caused by lacerations of bridging veins or cortical arteries. Alternatively, it has been hypothesized that chronic SDH arises from traumatic splitting of the cell layers in the dural border, leading to formation of a fragile neovascularized membrane that may rupture, causing a gradual accumulation of blood and fluid until this reaches a critical threshold and causes a pressure effect. Spontaneous SDHs are rare and occur secondary to coagulopathy disorders, often in the setting of a hematological malignancy. Patients with acute leukemia can present with SDH either at the time of initial diagnosis or later during the course of the illness [8]. A spontaneous SDH can be the presenting manifestation of acute leukemia and CML [8-11]. The reported incidence of SDH in patients with acute leukemia can range from 0.47% to 6% [12, 13].
Patients with asymptomatic SDH are managed conservatively; however, patients with clot thickness > 10 mm, midline shift > 5 mm, or rapid deterioration of neurological status require urgent surgical intervention. Surgical options include burr-hole evacuation or craniotomy. Any surgical intervention carries significant risks, including recurrent SDH (25%), infection, seizures, and intraoperative bleeding (1.7%) [14]. The risk of postoperative intracranial hematoma is significantly high (40%) in patients with thrombocytopenia, and the mortality rate can be as high as 20% [15]. While these risks can be to a certain extent mitigated with platelet transfusions, some patients are refractory to platelet therapy due to alloimmunization. Hence, leukemia patients with SDH in the setting of severe thrombocytopenia, coagulopathy, and disease-related immunosuppression pose a unique challenge. Given our experience in treating APL-related ICH [16], we initiated the APL treatment and placed our patient under observation, aware that a surgical procedure may have been ultimately required. Until now our standard approach for patients like this patient has been observation followed by surgical intervention when needed. When it became obvious that our patient was getting worse with progression of symptoms, increasing size of the SDH, and midline shift, instead of taking her to the operating room and based on the experience of a successful case we came across published in the medical literature, we opted to perform the MMA embolization.
MMA embolization is a minimally invasive endovascular approach. The goal is to devascularize the fragile subdural membranes. This has been proven to be highly effective in the management of chronic SDH [17, 18]. Given that MMA embolization is a minimally invasive approach, this novel therapy appears to be safe and effective in acute leukemia patients with acute SDH, given the complications associated with surgical management.
The safety of efficacy of MMA embolization in treating chronic SDH has been documented in multiple studies. Joyce et al published a multicenter retrospective study of outcomes of elderly patients with non-acute SDH treated with MMA embolization and found that > 90% of patients showed stabilization or improvement in SDH, and that only a minority (4.6-7.8%) required a salvage surgical intervention [19]. Ban et al [20] reported a prospective study (n = 72) from Korea of outcomes of patients with chronic SDH who underwent MMA embolization, compared with historical controls (n = 469) who were treated with conventional therapy. It was observed that treatment failure was significantly lower in the MMA embolization cohort (1.4% vs. 27.5%), as was the need for salvage surgical rescue (1.4% vs. 18%) [20]. Srivatsan et al published a systemic review and meta-analysis and noted that the SDH recurrence rate was significantly lower for MMA embolization than conventional therapy (2.1% vs. 27.7%) [21]. Kan et al reported a multicenter study of 154 consecutive patients with chronic SDH treated with MMA embolization and found that > 50% of patients had improvement in the size of the hematoma on imaging, and only 6.5% required further therapy for SDH [17]. Ironside et al published a systematic review and meta-analysis of 20 studies (n = 1,416) comparing MMA embolization with conventional therapies, and the recurrence rate of chronic SDH was significantly lower for MMA embolization than for conventional therapies (4.8% vs. 21%) [21]. Link et al published a single-center retrospective study of 60 patients with chronic SDH, and among patients treated with MMA embolization, only four (8.9%) required salvage surgery [18]. Lee et al reported outcomes of 22 oncology patients with refractory thrombocytopenia who were treated with MMA embolization, and 77% achieved a reduction in size of the hematoma, and only two required salvage surgery [23]. A summary of the studies is provided in Table 1 [17-22].
 Click to view | Table 1. Previous Reports of Middle Meningeal Artery (MMA) Embolization in cSDH Patients |
This experience together with recent data in oncological patients had given more visibility to this therapeutic strategy. Recently Maini et al published a case of a 25-year-old woman with APL and simultaneous cerebral venous thrombosis and SDH that was successfully managed with MMA embolization [24]. Burrows et al reported a case of a 35-year-old man with severe thrombocytopenia secondary to CML blast crisis and chronic SDH, who was successfully managed with MMA embolization [25]. To these two acute leukemia cases we now add our experience with these two additional cases of acute leukemia with an acute SDH successfully managed with MMA. After a review of the use of MMA in the literature in patients with chronic SDH and of the two reported cases, we have concluded that it is reasonable to follow the proposed treatment algorithm for chronic SDH developed by the Barrow Neurological Institute [26] (Supplementary Material 1, www.thejh.org).
Appropriate patient selection for patients in need of acute MMA embolization will be critical, especially in those with underlying cancer. Lee et al [23] reported successful treatment of chronic SDH with MMA embolization in the majority of patients; however, 73% died from oncology-related complications. The magnitude of benefit from MMA embolization for cancer patients can be marginal, especially in older patients with comorbidities and/or a high disease burden. However, in highly curable malignancies like APL, a minimally invasive approach to treat SDH is a valuable tool to add to the armamentarium available to the treating physician.
Conclusions
MMA embolization is a novel emerging therapeutic modality for the treatment of chronic SDH. Given that this is a minimally invasive intervention, it appears to be a quite promising approach in acute leukemia patients for whom conventional surgical procedures can be challenging in the setting of thrombocytopenia and coagulopathy.
| Supplementary Material | ▴Top |
Suppl 1. The proposed treatment algorithm for chronic SDH developed by the Barrow Neurological Institute.
Acknowledgments
The authors would like to thank Ms. Virginia Mohlere for editorial assistance.
Financial Disclosure
The authors have no funding to disclose.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
Informed consent was obtained from the patients for publication.
Author Contributions
All authors contributed to the gathering of data and final approval of the manuscript. BY drafted the manuscript; AR is the acute leukemia treating physician who critically reviewed the manuscript for important intellectual content, finalized all agreed-upon changes and submitted the final version.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Dayyani F, Mougalian SS, Naqvi K, Shan J, Ravandi F, Cortes J, Weinberg J, et al. Prediction model for mortality after intracranial hemorrhage in patients with leukemia. Am J Hematol. 2011;86(7):546-549.
doi pubmed pmc - Rogers LR. Cerebrovascular complications in patients with cancer. Semin Neurol. 2010;30(3):311-319.
doi pubmed - Velander AJ, DeAngelis LM, Navi BB. Intracranial hemorrhage in patients with cancer. Curr Atheroscler Rep. 2012;14(4):373-381.
doi pubmed - Pomeranz S, Naparstek E, Ashkenazi E, Nagler A, Lossos A, Slavin S, Or R. Intracranial haematomas following bone marrow transplantation. J Neurol. 1994;241(4):252-256.
doi pubmed - Stanworth SJ, Hudson CL, Estcourt LJ, Johnson RJ, Wood EM, investigators Ts. Risk of bleeding and use of platelet transfusions in patients with hematologic malignancies: recurrent event analysis. Haematologica. 2015;100(6):740-747.
doi pubmed pmc - Cornelissen LL, Kreuger AL, Caram-Deelder C, Middelburg RA, Kerkhoffs JLH, von dem Borne PA, Beckers EAM, et al. Thrombocytopenia and the effect of platelet transfusions on the occurrence of intracranial hemorrhage in patients with acute leukemia - a nested case-control study. Ann Hematol. 2021;100(1):261-271.
doi pubmed pmc - Stanworth SJ, Estcourt LJ, Powter G, Kahan BC, Dyer C, Choo L, Bakrania L, et al. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368(19):1771-1780.
doi pubmed - Xia G, Zhang W, Xiao J, Shi L, Zhang Y, Xue H. Chronic subdural hematoma caused by acute myeloblastic leukemia: a case report. Front Neurol. 2022;13:911195.
doi pubmed pmc - Abdulhamid MM, Li YM, Hall WA. Spontaneous acute subdural hematoma as the initial manifestation of chronic myeloid leukemia. J Neurooncol. 2011;101(3):513-516.
doi pubmed - Jain A, Prasad P, Chaudhry S, Gupta DK, Saluja S. Spontaneous acute subdural hematoma as an initial presentation of chronic myeloid leukemia. Indian J Hematol Blood Transfus. 2019;35(3):578-579.
doi pubmed pmc - Shi XY, Zhang JX, Tang ZX, Sun H, Shen Z. Severe spontaneous acute arterial subdural hematoma as an initial symptom of chronic myeloid leukemia. Br J Neurosurg. 2023;37(6):1721-1724.
doi pubmed - Chen CY, Tai CH, Cheng A, Wu HC, Tsay W, Liu JH, Chen PY, et al. Intracranial hemorrhage in adult patients with hematological malignancies. BMC Med. 2012;10:97.
doi pubmed pmc - Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine (Baltimore). 1985;64(1):16-35.
doi pubmed - Rauhala M, Helen P, Huhtala H, Heikkila P, Iverson GL, Niskakangas T, Ohman J, et al. Chronic subdural hematoma-incidence, complications, and financial impact. Acta Neurochir (Wien). 2020;162(9):2033-2043.
doi pubmed pmc - Chan KH, Mann KS, Chan TK. The significance of thrombocytopenia in the development of postoperative intracranial hematoma. J Neurosurg. 1989;71(1):38-41.
doi pubmed - Yohannan B, Cervoni-Curet F, Rios A. AML-156 early eathis in patients with newly diagnosed acute promyelocytic leukemia: a single-center retrpopsective study from a general hospital. Clin Lymphoma Myeloma Leuk. 2022;22(2):S219-S220.
- Kan P, Maragkos GA, Srivatsan A, Srinivasan V, Johnson J, Burkhardt JK, Robinson TM, et al. Middle meningeal artery embolization for chronic subdural hematoma: a multi-center experience of 154 consecutive embolizations. Neurosurgery. 2021;88(2):268-277.
doi pubmed - Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery. 2019;85(6):801-807.
doi pubmed - Joyce E, Bounajem MT, Scoville J, Thomas AJ, Ogilvy CS, Riina HA, Tanweer O, et al. Middle meningeal artery embolization treatment of nonacute subdural hematomas in the elderly: a multiinstitutional experience of 151 cases. Neurosurg Focus. 2020;49(4):E5.
doi pubmed - Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS, Han JH, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018;286(3):992-999.
doi pubmed - Srivatsan A, Mohanty A, Nascimento FA, Hafeez MU, Srinivasan VM, Thomas A, Chen SR, et al. Middle meningeal artery embolization for chronic subdural hematoma: meta-analysis and systematic review. World Neurosurg. 2019;122:613-619.
doi pubmed - Ironside N, Nguyen C, Do Q, Ugiliweneza B, Chen CJ, Sieg EP, James RF, et al. Middle meningeal artery embolization for chronic subdural hematoma: a systematic review and meta-analysis. J Neurointerv Surg. 2021;13(10):951-957.
doi pubmed - Lee S, Srivatsan A, Srinivasan VM, Chen SR, Burkhardt JK, Johnson JN, Raper DMS, et al. Middle meningeal artery embolization for chronic subdural hematoma in cancer patients with refractory thrombocytopenia. J Neurosurg. 2022;136(5):1273-1277.
doi pubmed - Maini K, Afzal F, Giurgiutiu DV, Rahimi SY, Shah M, Switzer JA, Vale FL, et al. Concomitant central venous sinus thrombosis and subdural hematoma in acute promyelocytic leukemia: middle meningeal artery embolization enables safe anticoagulation. Illustrative case. J Neurosurg Case Lessons. 2021;1(23):CASE2080.
doi pubmed pmc - Burrows EJ, Lee SC, Bangash OK, Phillips TJ, Lee S. Middle meningeal artery embolization for chronic subdural hematoma in a young patient with refractory thrombocytopenia secondary to leukemia: more evidence for a paradigm shift? Illustrative case. J Neurosurg Case Lessons. 2023;5(9):CASE22273.
doi pubmed pmc - Catapano JS, Nguyen CL, Wakim AA, Albuquerque FC, Ducruet AF. Middle meningeal artery embolization for chronic subdural hematoma. Front Neurol. 2020;11:557233.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


