| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 13, Number 3, June 2024, pages 128-136
Unraveling the Rare Entity of KIT D816V-Negative Systemic Mastocytosis
Ruah Alyamanya, Chams Alkhalaf Albachirb, Sarah Alsalehb, Alaa Hamadb, Sameeha Kaiser Abdulwalib, Ahmad S. Alotaibia, Syed Osman Ahmeda, Mansour Alfayeza, b, c
aDepartment of Hematology, Stem Cell Transplant and Cellular Therapy, Oncology Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 11211, Saudi Arabia
bCollege of Medicine, Alfaisal University, Riyadh, Saudi Arabia
cCorresponding Author: Mansour Alfayez, Department of Hematology, Stem Cell Transplant and Cellular Therapy, Oncology Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 11211, Saudi Arabia
Manuscript submitted April 16, 2024, accepted May 28, 2024, published online June 28, 2024
Short title: KIT D816V-Negative Systemic Mastocytosis
doi: https://doi.org/10.14740/jh1279
| Abstract | ▴Top |
Systemic mastocytosis (SM) is a rare type of myeloproliferative neoplasm characterized by abnormal proliferation and infiltration of different tissue by clonal mast cells. The uncontrolled proliferation and activation of mast cells trigger the release of vasoactive and inflammatory mediators, resulting in a cascade of systemic symptoms. Around 95% of SM arise from a gain-of-function mutation at the KIT gene, specifically at codon 816, which highlights its essential role in SM and makes it an attractive target for therapy. Although KIT-negative SM is exceptionally rare, the increased number of cases documented in the literature makes it an intriguing dimension of this disorder. The reported clinical manifestations of KIT-negative SM are widely variable, but many are similar to KIT-positive SM. KIT-targeted therapeutic options have been a game-changer in KIT-positive SM, however their role in KIT-negative SM remains controversial. This report aimed to further understand KIT-negative SM by presenting two cases of KIT-negative SM, one of which was responsive to KIT-targeted therapy, and analyzing reported cases in the existing literature.
Keywords: Systemic mastocytosis; KIT-negative systemic mastocytosis; Tyrosine kinase inhibitor; Imatinib; Avapritinib; Midostaurin
| Introduction | ▴Top |
Mast cell disorders represent a category of myeloproliferative neoplasms characterized by aberrant clonal mast cell proliferation [1, 2]. These abnormal mast cells infiltrate various tissues and get activated, leading to clinical manifestations mimicking allergic reactions without an identifiable allergic trigger [3, 4]. The International Consensus Classification (ICC) and World Health Organization (WHO) recently updated their classification of mastocytosis. It classified mast cell disorders into cutaneous mastocytosis, systemic mastocytosis (SM), and mast cell sarcoma (MCS), with further subclassifications depending on the extent of involvement within each category (Table 1) [1, 5-10]. The diagnostic criteria for SM in the WHO fifth edition and in ICC were revised to require a major criterion with one minor or at least three minor criteria (Table 2) [1, 8-11].
 Click to view | Table 1. The WHO Fifth Edition (WHO 5th) and the ICC Classifications of Mastocytosis |
 Click to view | Table 2. The WHO Fifth Edition (WHO 5th) and the ICC Diagnostic Criteria for SM |
SM (SM) is a rare type of mast cell disorder characterized by the infiltration of various tissues, including but not limited to the bone marrow, gastrointestinal organs, and other extracutaneous tissue, by dysregulated mast cells [1, 12]. Although identifying KIT-activating gene mutation, specifically at codon 816, is considered a fundamental part of the diagnosis and is one of the four minor criteria according to the ICC diagnostic criteria for SM, its negativity does not exclude the diagnosis [1, 13]. Few patients do not reveal a KIT mutation; however, these cases are rare [12]. In this case report, we report two cases of KIT-negative SM and a review of the cases reported in the literature. We aimed to enhance our understanding of this exceedingly rare entity’s distinctive features, the diagnosis, and management.
| Case Reports | ▴Top |
Case 1
A 49-year-old previously healthy female was referred to our care with a 4-year history of painless and nonpruritic cutaneous eruptions affecting her upper and lower extremities, as well as the abdomen. These eruptions occurred spontaneously, without identified triggers. In the year preceding her hospital visit, the patient experienced unexplained episodes of dizziness accompanied by nausea and vomiting. The diagnostic investigation revealed thrombocytopenia (C-finding) and a high serum tryptase level (157 µg/L). Computed tomography (CT) imaging showed splenomegaly (B-finding) and multiple lytic lesions (C-finding) (Table 3) [1, 9]. A subsequent bone marrow biopsy disclosed 70% cellularity, with 40% mast cells, consistent with SM. Immunostaining was positive for CD117, CD68, and tryptase, while molecular testing from the peripheral blood using polymerase chain reaction (PCR) to amplify exons 8, 9, 11, 13, and 17 of c-KIT was negative. Attempts to get a sample for the bone marrow were unsuccessful as they resulted in a dry tap. There was no evidence of other concomitant hematological malignancy on bone marrow examination. Positron emission tomography (PET)/CT scan illustrated fluorodeoxyglucose (FDG)-avid generalized skeletal sclerosis attributable to the underlying SM. The patient was started on imatinib along with symptomatic treatment such as topical clobetasol, famotidine, and loratadine, which led to improvement in the skin lesions, although she reported muscle aches, which were attributed to medication-related adverse effects. The patient is presently well maintained on a regimen of 300 mg of imatinib daily 2 years from the initial presentation.
 Click to view | Table 3. The WHO Fifth Edition Summarizing the Clinical Findings of SM, Classified Into B-Findings and C-Findings (Adapted From Valent et al [1, 9]) |
Case 2
A 40-year-old male, known to have bronchial asthma, sought medical attention for recurrent anaphylactic episodes marked by syncope, skin manifestations, respiratory distress, and gastrointestinal symptoms. The clinical presentation, initially triggered by physical exertion and later associated with specific dietary factors, prompted comprehensive diagnostic investigations. These episodes started at the age of 30 years and manifested as sudden-onset headaches, palpitations, and flushing, followed by an episode of brief loss of consciousness. The evaluation revealed eosinophilia, prompting bone marrow biopsy, which revealed indolent SM, demonstrating multifocal infiltration by atypical mast cells expressing tryptase, CD117, and CD25. Cytogenetic studies were negative for PDGFR alpha and beta rearrangements, and molecular testing from the bone marrow sample using PCR to amplify exons 8, 9, 11, 13, and 17 of c-KIT was negative, as well as testing of the KIT mutation through the myeloid next-generation sequencing (NGS) panel on the bone marrow samples, returned negative results. There was no evidence of an associated myeloid neoplasm on bone marrow review. Therapy with midostaurin was started, while continuing on supportive therapy which included montelukast, cetirizine, and inhaled intranasal and systemic steroids (prednisolone 5 mg daily), resulting in a significant reduction in symptom frequency. However, subsequent challenges in medication availability led to sequential transitions to dasatinib, which was changed later to imatinib due to poor response, and ultimately avapritinib after developing another anaphylactic reaction 9 months after starting imatinib. The patient is well managed with avapritinib and has a complete clinical response; however, he developed hair and skin depigmentation, attributed to avapritinib treatment (Fig. 1).
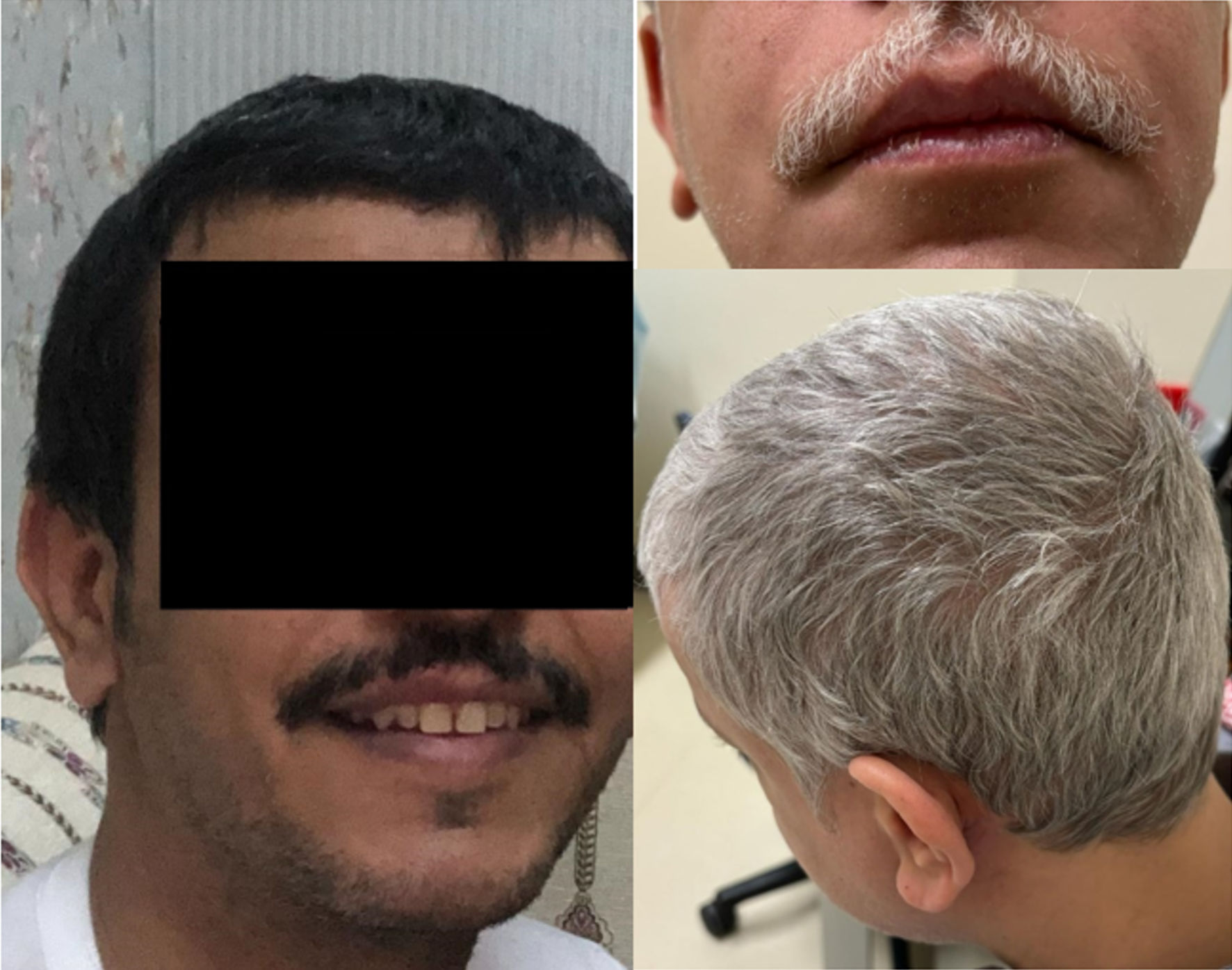 Click for large image | Figure 1. Case 2: skin and hair color pre (left) and post (right) avapritinib treatment. Wild-type KIT signaling is involved in melanogenesis and hair pigmentation, and inhibition can result in hair depigmentation and lightning of the skin through a temporary melanocyte dysfunction. Avapritinib skin and hair changes were reported in 6% to 21% of treated patients. |
| Discussion | ▴Top |
SM represents a rare and aggressive entity within the spectrum of mast cell disorders, featuring further subclassifications according to the ICC that vary in severity, including indolent SM (incorporating bone marrow mastocytosis), smoldering SM, aggressive SM, SM with an associated hematologic neoplasm (AHN-SM), and mast cell leukemia (MCL) (Table 1) [1, 8-10].
Mastocytosis is often linked to somatic gain-of-function point mutations in the KIT gene, namely at the D816V domain, where aspartic acid is replaced by valine, resulting in unregulated proliferation, amplified growth, and increased survival of mast cells [14, 15]. Various cellular entities, including mast cells, hematopoietic progenitor cells, germ cells, melanocytes, and interstitial cells of Cajal within the gastrointestinal tract, manifest the expression of KIT, a type III receptor belonging to the tyrosine kinase family encoded by a 21-exon containing gene located on chromosome 4q12 [9, 16-19]. In normal circumstances, the KIT gene is downregulated upon the maturation of all hematopoietic progenitor cells, except for mast cells. Mast cell surfaces express KIT genes at high levels. However, the stem cell factor, a ligand for KIT, controls the mast cell activity [9, 15]. Other less common mutations observed in advanced SM encompass, yet are not confined to, TET2, SRSF2, ASXL1, RUNX1, JAK2, N/KRAS, CBL, and EZH2 [13, 20-22]. Although KIT mutations are prevalent, occurring in > 95% of SM cases, their uniform presence is not ubiquitous [12, 23]. The occurrence of KIT-negative cases of SM is notably rare [9, 24]. However, their prevalence has gained increased recognition over the years, as evidenced by the cases reported in the literature (Table 4) [14, 25-28] and those presented in this paper. Research is still being done to understand better the complex interactions between different mutations, their capacity to change mast cells, and their influence on clinical manifestations and treatment of mastocytosis [9]. Individuals with SM manifest symptoms primarily related to the infiltration of different tissues by mast cells and their activation, which leads to the release of vasoactive mediators and cytokines [3]. The clinical manifestations include episodic flushing, diarrhea, hypotension, osteoporosis, and abdominal pain [4, 14]. In the context of KIT-negative SM, there is not enough evidence on the specific features seen in this entity; however, there is evidence expressing its association with advanced SM subtypes, such as MCL and MSC [12, 29]. According to the cases we have reported and reviewed in the literature, the clinical symptoms seem similar to those of KIT-positive SM (Table 3) [1, 9]. Additional features reported in the sparse cases available in the literature include dizziness, nausea, vomiting, headache, fatigue, weight loss, memory problems, insomnia, exertional dyspnea, joint pain, lower gastrointestinal bleeding, and compression fractures [14, 25-28]. By assessing both our reported KIT-negative SM cases and those documented in the literature, we can see that there appears to be a noticeable diversity in clinical manifestations. This spectrum includes a range of symptom types and varying degrees of severity, collectively emphasizing the notable variability in this condition (Table 3) [1, 9].
 Click to view | Table 4. Clinical and Pathologic Comparison of Our SM Cases and Cases in the Literature |
Detecting KIT-activating point mutations is typically done using molecular biology techniques, precisely PCR in conjunction with DNA sequencing [9, 15, 30]. The ability to detect the KIT-D816V mutation relies on the sensitivity of the test used and the concentration of mast cells in the sample [31]. KIT-D816V testing from whole blood has been reported to have high specificity but limited sensitivity [32]. To enhance the sensitivity of this testing, it has been recommended that the test be performed on purified cell groups, including abnormal mast cells, eosinophils, basophils, and monocytes [32]. However, targeting the affected mast cells or other cell groups makes testing challenging, and this is further complicated by the inconsistency of mast cell infiltration in cases of SM. Several attempts have been made to enhance the sensitivity of detecting KIT mutations in SM [30]. Kristensen et al developed a quantitative and sensitive allele-specific real-time quantitative polymerase chain reaction (qPCR) assay to detect the KIT-D816V mutation [30]. This test detected the mutation in 19 out of 20 SM patients at low levels, as low as 0.03% in bone marrow mononuclear cells [30]. In cases of a negative KIT testing result, applying refined methodologies becomes essential for assessing the results’ reliability, particularly when the mutation detection limit exceeds the proportion of mast cells within the sample, often due to the island-like aggregation of mast cells in the bone marrow [30]. Nevertheless, the genuinely negative KIT mutation is believed to occur in some patients with SM. Further refinement and advancement of methodologies and investigations are needed to improve KIT mutation detection and accurately diagnose KIT-negative SM. NGS can enhance the ability to detect KIT and non-KIT mutations and quantify the variant allele frequency (VAF) of the KIT-D816V mutation [33, 34]. A limitation of our study is the unavailability of the most advanced tools for detecting KIT mutations, which may have led to false-negative results in the cases we reported.
The therapeutic approach to SM depends on the severity of symptoms. Therapy can range from vigilant monitoring to cytoreductive therapy and, in rare and more aggressive cases, stem cell transplantation [9, 34]. Treatment modalities include symptom alleviation, mast cell reduction strategies for disease modification, and the implementation of supportive measures [9].
Symptomatic control involves the regulation of vasoactive mediators and cytokines, achieved through administration of H1 and H2 antihistamines, anti-leukotrienes, and nonsteroidal anti-inflammatory drugs (nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin) [9, 34, 35]. Omalizumab, a recombinant humanized monoclonal antibody, improves mastocytosis symptoms by inhibiting immunoglobulin E (IgE) binding to the high-affinity IgE receptor (RI) on mast cells’ surface. Its efficacy is particularly seen in patients experiencing recurrent anaphylaxis [9, 34, 36]. Despite its symptomatic benefits, the Food and Drug Administration (FDA) has not yet approved omalizumab for mastocytosis [9, 36]. Disodium cromoglycate is an organic sodium salt used to treat asthma. It has improved tryptase levels and symptoms in KIT-D816V-negative SM cases [27]. It functions as a mast cell stabilizer and has an anti-inflammatory effect. In the report by Conde-Fernandes et al, the patient turned out to have a different type of KIT mutation, known as the KIT-V560G mutation [27].
Advanced cases require additional interventions, including cytoreductive agents, for example, hydroxyurea, which causes non-targeted myelosuppression, purine analogs such as cladribine, and interferon-α [4, 9, 37-39]. Cladribine has demonstrated efficacy across all the subtypes of SM, with the additional advantage being time-constrained [9, 38]. Interferon-α is less frequently used but may be valuable in areas with modest resources [9, 39]. However, with the rapid evolution in the medical field, advancements in targeted therapies have emerged, which has increased their appeal. The high prevalence of KIT mutations in SM makes it an attractive therapeutic target [40, 41]. Examples of kinase inhibitors that selectively target KIT mutations include avapritinib, approved as first-line therapy for indolent and advanced SM [42-45]. Regarding cases of true KIT-negative SM, it is theorized that therapies that selectively target KIT mutations are less effective when compared to their impact in cases of KIT-positive SM. Therefore, pursuing treatments with alternative mechanisms of action beyond KIT mutation targeting is considered a viable therapeutic strategy when managing such cases. That being said, there is evidence demonstrating the efficacy of avapritinib in cases of KIT-negative SM [25]. This observation is highlighted in some of the cases reported, including one of ours (Table 3) [1, 9]. Gotlib et al explored the efficacy of midostaurin, a potent multi-kinase inhibitor, in cases of advanced SM and reported a response rate of up to 60%, irrespective of KIT-mutation status [46, 47]. Alternatively, tyrosine kinase inhibitors (TKIs) such as imatinib, nilotinib, and dasatinib are used in cases of KIT-negative SM. Interestingly, KIT-positive SM patients respond poorly to various TKIs, especially to imatinib, as KIT-D816V mutations result in primary resistance [48-51]. Nonetheless, there has been significant heterogeneity in the responsiveness to different TKIs across the reported cases of KIT-negative SM (Table 3) [1, 9].
Multiple medications are being investigated for efficacy in SM, including bezuclastinib, an orally administered potent and selective type I TKI targeting multiple loci, including D816V [52-54]. BLU-263 is an orally administered selective inhibitor targeting KIT D816V [55, 56]. Masitinib is a TKI exhibiting activity against wild-type KIT, Lyn, and Fyn kinases [57].
Supportive interventions are vital to managing SM. These measures include avoiding triggers such as aspirin, contrast dyes, anesthesia, narcotics, and alcohol, as well as preventing and managing osteopenia/osteoporosis [9, 34].
The prognosis of SM depends on different factors, including age, cytopenias, the WHO classification-defined subtype of SM, biochemical markers, and mutational profile [58-60]. Multiple prognostic scoring systems are used for advanced and non-advanced SM, e.g., REMA, IPSM, GPS, MARS, and MAPS scoring systems. These scoring systems rely on parameters such as age, blood counts, serum tryptase, β2-microglobulin, alkaline phosphatase, and the mutational profile [20, 21, 34, 61, 62]. The presence of non-KIT mutations, such as SRSF2, ASXL1, RUNX1, and EZH2, has been associated with advanced SM subtypes and inferior prognosis [12, 20-22, 34]. Although a KIT-D816V VAF of > 10% has been linked with a higher tumor burden [63, 64], the prognosis of patients with negative KIT-D816V mutations remains vague. In cases of KIT-D816V-negative SM, these prognostic scores are controversial.
A report by Naumann et al concluded that the conventional scoring systems used in SM cannot be applied in cases of KIT-negative SM and have linked the negativity of KIT in SM with an inferior response to treatment and overall survival [12]. However, in AHN-SM, patients with KIT-D816V-negative mutation had a lower burden of mast cells with the domination of the AHN, which resulted in a better overall survival [12]. In this review, we have reported a case of KIT-D816V-negative SM with an excellent response to avapritinib, along with the reported case by Azad et al (Table 3) [1, 9, 25], which can serve as an initiation point for further comprehensive cohort studies to assess the effect of avapritinib on KIT-D816V-negative SM. The prognostic implications of the KIT D816V mutation require additional investigations to thoroughly establish its influence on overall survival, disease-free survival, and quality of life [14, 60].
Conclusions
SM is a rare disease, and the KIT-D816V-negative SM subset accounts for less than 5% of all cases. This exceedingly rare entity’s specific clinical manifestations, treatment, and prognosis are poorly understood. Further and more comprehensive research is needed to expand our understanding of KIT-D816V-negative SM and determine the most appropriate management and prognosis.
Acknowledgments
None to declare.
Financial Disclosure
This report is not funded by a specific grant.
Conflict of Interest
Mansour Alfayez: Honoraria: Johnson & Johnson, Pfizer, Astellas, Novartis, Amgen, AstraZeneca, AbbVie; Advisory board: Johnson & Johnson, Biologix, Eli Lilly; Research support: Abbvie, AstraZeneca. Other authors declare no conflict of interest with this manuscript.
Informed Consent
Informed consents were obtained.
Author Contributions
RA, CAA, SA, AH, and SKA compiled and summarized the data. RA, SOA and MA treated the patient and wrote the article. All authors contributed, reviewed, and edited the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Valent P, Akin C, Hartmann K, Alvarez-Twose I, Brockow K, Hermine O, Niedoszytko M, et al. Updated diagnostic criteria and classification of mast cell disorders: a consensus proposal. Hemasphere. 2021;5(11):e646.
doi pubmed pmc - Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419-432.
doi pubmed - George TI, Horny HP. Systemic mastocytosis. Hematol Oncol Clin North Am. 2011;25(5):1067-1083.
doi pubmed - Fletcher L, Borate U. Novel approaches for systemic mastocytosis. Curr Opin Hematol. 2019;26(2):112-118.
doi pubmed - Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703-1719.
doi pubmed pmc - Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129(11):1420-1427.
doi pubmed pmc - Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405.
doi pubmed - Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, Wang SA, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200-1228.
doi pubmed pmc - Pardanani A. Systemic mastocytosis in adults: 2023 update on diagnosis, risk stratification and management. Am J Hematol. 2023;98(7):1097-1116.
doi pubmed - Gaik C, Wiesmann T. Systemic mastocytosis. Anasthesiologie und Intensivmedizin. 2021;62(10):S266-S282.
doi - Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, Marone G, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25(7):603-625.
doi pubmed - Naumann N, Rudelius M, Lubke J, Christen D, Bresser J, Sotlar K, Metzgeroth G, et al. Poor Applicability of Currently Available Prognostic Scoring Systems for Prediction of Outcome in KIT D816V-Negative Advanced Systemic Mastocytosis. Cancers (Basel). 2024;16(3):593.
doi pubmed pmc - Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, Metcalfe DD. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92(23):10560-10564.
doi pubmed pmc - Caceres-Nazario B, Caceres-Perkins W, Tasso D, Calderon-Alicea E, Conde-Sterling D, Arroyo-Portela N. Case report: unusual manifestation of KIT negative systemic mastocytosis. Am J Hematol Oncol. 2016;12(12):24-27.
pubmed pmc - Valent P. Diagnosis and management of mastocytosis: an emerging challenge in applied hematology. Hematology Am Soc Hematol Educ Program. 2015;2015:98-105.
doi pubmed - Garcia-Montero AC, Jara-Acevedo M, Teodosio C, Sanchez ML, Nunez R, Prados A, Aldanondo I, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108(7):2366-2372.
doi pubmed - Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, McClure RF, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113(23):5727-5736.
doi pubmed - Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13(3):205-220.
doi pubmed - Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, Kristensen TK, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015;29(6):1223-1232.
doi pubmed pmc - Munoz-Gonzalez JI, Alvarez-Twose I, Jara-Acevedo M, Zanotti R, Perkins C, Jawhar M, Sperr WR, et al. Proposed global prognostic score for systemic mastocytosis: a retrospective prognostic modelling study. Lancet Haematol. 2021;8(3):e194-e204.
doi pubmed - Pardanani A, Shah S, Mannelli F, Elala YC, Guglielmelli P, Lasho TL, Patnaik MM, et al. Mayo alliance prognostic system for mastocytosis: clinical and hybrid clinical-molecular models. Blood Adv. 2018;2(21):2964-2972.
doi pubmed pmc - Jawhar M, Schwaab J, Hausmann D, Clemens J, Naumann N, Henzler T, Horny HP, et al. Splenomegaly, elevated alkaline phosphatase and mutations in the SRSF2/ASXL1/RUNX1 gene panel are strong adverse prognostic markers in patients with systemic mastocytosis. Leukemia. 2016;30(12):2342-2350.
doi pubmed - Garcia-Montero AC, Jara-Acevedo M, Alvarez-Twose I, Teodosio C, Sanchez-Munoz L, Muniz C, Munoz-Gonzalez JI, et al. KIT D816V-mutated bone marrow mesenchymal stem cells in indolent systemic mastocytosis are associated with disease progression. Blood. 2016;127(6):761-768.
doi pubmed - Lim KH, Pardanani A, Tefferi A. KIT and mastocytosis. Acta Haematol. 2008;119(4):194-198.
doi pubmed - Azad F, Zhang J, Wang E. Avapritinib for the treatment of KIT mutation-negative systemic mastocytosis. Proc (Bayl Univ Med Cent). 2023;36(1):81-82.
doi pubmed pmc - Sakane-Ishikawa E, Kodaka T, Tsunemine H, Itoh K, Akasaka H, Kusama T, Imaizumi K, et al. Eosinophilia and bone lesion as clinical manifestations of aggressive systemic mastocytosis. J Clin Exp Hematop. 2013;53(3):207-213.
doi pubmed - Conde-Fernandes I, Sampaio R, Moreno F, Palla-Garcia J, Teixeira MDA, Freitas I, Neves E, et al. Systemic mastocytosis with KIT V560G mutation presenting as recurrent episodes of vascular collapse: response to disodium cromoglycate and disease outcome. Allergy Asthma Clin Immunol. 2017;13:21.
doi pubmed pmc - Savini P, Rondoni M, Poletti G, Lanzi A, Quercia O, Soverini S, De Benedittis C, et al. Serum total tryptase level confirms itself as a more reliable marker of mast cells burden in mast cell leukaemia (aleukaemic variant). Case Rep Hematol. 2015;2015:737302.
doi pubmed pmc - Gonzalez-Lopez O, Munoz-Gonzalez JI, Orfao A, Alvarez-Twose I, Garcia-Montero AC. Comprehensive analysis of acquired genetic variants and their prognostic impact in systemic mastocytosis. Cancers (Basel). 2022;14(10):2487.
doi pubmed pmc - Kristensen T, Vestergaard H, Moller MB. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J Mol Diagn. 2011;13(2):180-188.
doi pubmed pmc - Tefferi A, Skoda R, Vardiman JW. Myeloproliferative neoplasms: contemporary diagnosis using histology and genetics. Nat Rev Clin Oncol. 2009;6(11):627-637.
doi pubmed - Navarro-Navarro P, Alvarez-Twose I, Perez-Pons A, Henriques A, Mayado A, Garcia-Montero AC, Sanchez-Munoz L, et al. KITD816V mutation in blood for the diagnostic screening of systemic mastocytosis and mast cell activation syndromes. Allergy. 2023;78(5):1347-1359.
doi pubmed - Jawhar M, Schwaab J, Schnittger S, Meggendorfer M, Pfirrmann M, Sotlar K, Horny HP, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia. 2016;30(1):136-143.
doi pubmed - Lee HJ. Recent advances in diagnosis and therapy in systemic mastocytosis. Blood Res. 2023;58(S1):96-108.
doi pubmed pmc - Valent P, Akin C, Escribano L, Fodinger M, Hartmann K, Brockow K, Castells M, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37(6):435-453.
doi pubmed - Jendoubi F, Gaudenzio N, Gallini A, Negretto M, Paul C, Bulai Livideanu C. Omalizumab in the treatment of adult patients with mastocytosis: A systematic review. Clin Exp Allergy. 2020;50(6):654-661.
doi pubmed - Lim KH, Pardanani A, Butterfield JH, Li CY, Tefferi A. Cytoreductive therapy in 108 adults with systemic mastocytosis: Outcome analysis and response prediction during treatment with interferon-alpha, hydroxyurea, imatinib mesylate or 2-chlorodeoxyadenosine. Am J Hematol. 2009;84(12):790-794.
doi pubmed - Lubke J, Schwaab J, Naumann N, Horny HP, Weiss C, Metzgeroth G, Kreil S, et al. Superior efficacy of midostaurin over cladribine in advanced systemic mastocytosis: a registry-based analysis. J Clin Oncol. 2022;40(16):1783-1794.
doi pubmed - Kluin-Nelemans HC, Jansen JH, Breukelman H, Wolthers BG, Kluin PM, Kroon HM, Willemze R. Response to interferon alfa-2b in a patient with systemic mastocytosis. N Engl J Med. 1992;326(9):619-623.
doi pubmed - Mayerhofer M, Gleixner KV, Hoelbl A, Florian S, Hoermann G, Aichberger KJ, Bilban M, et al. Unique effects of KIT D816V in BaF3 cells: induction of cluster formation, histamine synthesis, and early mast cell differentiation antigens. J Immunol. 2008;180(8):5466-5476.
doi pubmed pmc - Zappulla JP, Dubreuil P, Desbois S, Letard S, Hamouda NB, Daeron M, Delsol G, et al. Mastocytosis in mice expressing human Kit receptor with the activating Asp816Val mutation. J Exp Med. 2005;202(12):1635-1641.
doi pubmed pmc - Evans EK, Hodous BL, Gardino AK, et al. Abstract 791: BLU-285, the first selective inhibitor of PDGFRα D842V and KIT Exon 17 mutants. Cancer Res. 2015;75(15_Supplement):791-791.
doi - Evans E, Hodous B, Gardino A, et al. First selective KIT D816V inhibitor for patients with systemic mastocytosis. Blood. 2014;124:3217.
doi - Evans EK, Gardino AK, Kim JL, Hodous BL, Shutes A, Davis A, Zhu XJ, et al. A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci Transl Med. 2017;9(414):eaao1690.
doi pubmed - DeAngelo DJ, Radia DH, George TI, Robinson WA, Quiery AT, Drummond MW, Bose P, et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: the phase 1 EXPLORER trial. Nat Med. 2021;27(12):2183-2191.
doi pubmed pmc - Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, Awan FT, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530-2541.
doi pubmed - Chandesris MO, Damaj G, Lortholary O, Hermine O. Clinical potential of midostaurin in advanced systemic mastocytosis. Blood Lymphat Cancer. 2017;7:25-35.
doi pubmed pmc - Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Casteran N, Borge L, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4(9):e7258.
doi pubmed pmc - Growney JD, Clark JJ, Adelsperger J, Stone R, Fabbro D, Griffin JD, Gilliland DG. Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412. Blood. 2005;106(2):721-724.
doi pubmed pmc - Hochhaus A, Baccarani M, Giles FJ, le Coutre PD, Muller MC, Reiter A, Santanastasio H, et al. Nilotinib in patients with systemic mastocytosis: analysis of the phase 2, open-label, single-arm nilotinib registration study. J Cancer Res Clin Oncol. 2015;141(11):2047-2060.
doi pubmed pmc - Verstovsek S, Tefferi A, Cortes J, O'Brien S, Garcia-Manero G, Pardanani A, Akin C, et al. Phase II study of dasatinib in Philadelphia chromosome-negative acute and chronic myeloid diseases, including systemic mastocytosis. Clin Cancer Res. 2008;14(12):3906-3915.
doi pubmed pmc - DeAngelo DJ, Pullarkat VA, Piris-Villaespesa M, et al. Preliminary safety and efficacy from apex, a phase 2 study of bezuclastinib (CGT9486), a novel, highly selective, potent KIT D816V tyrosine kinase inhibitor, in adults with advanced systemic mastocytosis (AdvSM). Blood. 2022;140(Supplement 1):1512-1513.
doi - Siebenhaar F, Gotlib J, Deininger MW, et al. A 3-part, phase 2 study of bezuclastinib (CGT9486), an oral, selective, and potent KIT D816V inhibitor, in adult patients with nonadvanced systemic mastocytosis (NonAdvSM). Blood. 2021;138(Supplement 1):3642-3642.
doi - Akin C, Siebenhaar F, Deininger MW, et al. Summit: a 3-part, phase 2 study of Bezuclastinib (CGT9486), an oral, selective, and potent KIT D816V inhibitor, in adult patients with nonadvanced systemic mastocytosis (NonAdvSM). Blood. 2022;140(Supplement 1):6838-6839.
doi - DeAngelo DJ, Reiter A, George TI, et al. AZURE: A phase 1/2 study of Blu-263 as monotherapy and in combination with azacitidine in patients with advanced systemic mastocytosis. Blood. 2022;140(Supplement 1):6877-6878.
doi - Castells M, Si TD, Bhavsar V, He K, Akin C. A phase 2/3 study of BLU-263 in patients with indolent systemic mastocytosis or monoclonal mast cell activation syndrome. Journal of Allergy and Clinical Immunology. 2022;149(2):AB221.
doi - Lortholary O, Chandesris MO, Bulai Livideanu C, Paul C, Guillet G, Jassem E, Niedoszytko M, et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomised, placebo-controlled, phase 3 study. Lancet. 2017;389(10069):612-620.
doi pubmed pmc - Jawhar M, Schwaab J, Naumann N, Horny HP, Sotlar K, Haferlach T, Metzgeroth G, et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood. 2017;130(2):137-145.
doi pubmed - Munoz-Gonzalez JI, Jara-Acevedo M, Alvarez-Twose I, Merker JD, Teodosio C, Hou Y, Henriques A, et al. Impact of somatic and germline mutations on the outcome of systemic mastocytosis. Blood Adv. 2018;2(21):2814-2828.
doi pubmed pmc - Jawhar M, Dohner K, Kreil S, Schwaab J, Shoumariyeh K, Meggendorfer M, Span LLF, et al. KIT D816 mutated/CBF-negative acute myeloid leukemia: a poor-risk subtype associated with systemic mastocytosis. Leukemia. 2019;33(5):1124-1134.
doi pubmed pmc - Munoz-Gonzalez JI, Alvarez-Twose I, Jara-Acevedo M, Henriques A, Vinas E, Prieto C, Sanchez-Munoz L, et al. Frequency and prognostic impact of KIT and other genetic variants in indolent systemic mastocytosis. Blood. 2019;134(5):456-468.
doi pubmed - Sperr WR, Kundi M, Alvarez-Twose I, van Anrooij B, Oude Elberink JNG, Gorska A, Niedoszytko M, et al. International prognostic scoring system for mastocytosis (IPSM): a retrospective cohort study. Lancet Haematol. 2019;6(12):e638-e649.
doi pubmed pmc - Hoermann G, Gleixner KV, Dinu GE, Kundi M, Greiner G, Wimazal F, Hadzijusufovic E, et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy. 2014;69(6):810-813.
doi pubmed pmc - Erben P, Schwaab J, Metzgeroth G, Horny HP, Jawhar M, Sotlar K, Fabarius A, et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann Hematol. 2014;93(1):81-88.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


