| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Short Communication
Volume 9, Number 3, September 2020, pages 71-78
Clinical Features of Plasmablastic Lymphoma: Case Series From an Asian Tertiary Cancer Center and Literature Review
Daniel Ren Yi Yapa, Grace Fangmin Tanb, Esther Wei Yin Changb, Valerie Shiwen Yangb, Eileen Yi Ling Poonb, c, Nagavalli Somasundaramb, c, Mohamad Faridb, c, d, Tiffany Tangb, c, d, Miriam Taob, c, d, Soon Thye Limb, c, d, Jason Yongsheng Chanb, d, e, f
aLee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore
bDivision of Medical Oncology, National Cancer Centre Singapore, Singapore, Singapore
cSingHealth Duke-NUS Blood Cancer Centre, Singapore, Singapore
dDuke-NUS Medical School, Singapore, Singapore
eCancer Science Institute of Singapore, National University of Singapore, Singapore, Singapore
fCorresponding Author: Jason Yongsheng Chan, Division of Medical Oncology, National Cancer Centre Singapore, 11 Hospital Drive, Singapore 169610, Singapore
Manuscript submitted May 16, 2020, accepted June 18, 2020, published online August 14, 2020
Short title: Features of Asian Plasmablastic Lymphoma
doi: https://doi.org/10.14740/jh672
| Abstract | ▴Top |
Background: Plasmablastic lymphoma (PBL) is an aggressive subtype of mature B-cell non-Hodgkin lymphoma. Given its rarity, there remains a lack of clinicopathological data to guide its management, particularly on Asian patients.
Methods: We conducted a retrospective chart review of 10 patients diagnosed with PBL at the National Cancer Centre Singapore and performed a literature review of similar studies on Asian cohorts.
Results: Most patients were male (n = 9), with median age at diagnosis of 55 years (range, 33 - 91 years). Seven (70%) patients were considered to be immunocompromised. In the overall cohort, the median overall survival (OS) was 19.4 months with 5-year survival estimates given at 60% and 36% for OS and progression-free survival (PFS), respectively. At diagnosis, patients with HIV/AIDS (n = 5) were younger compared to others (median, 43 vs. 61 years; P = 0.0278), had greater number of nodal site involvement (median, 6 vs. 0; P = 0.0333), and higher international prognostic index (IPI) scores (P = 0.034 for trend). Amongst different chemotherapy used, etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin (EPOCH)-based regimens (n = 6) elicited prominent complete response rates (83%) and led to durable responses even in the setting of advanced stage, high-risk IPI score and immunodeficiency.
Conclusions: In conclusion, our study describes the features of PBL in an Asian cohort and highlights disease features unique to HIV-associated PBL.
Keywords: Epstein-Barr virus; Immunodeficiency; HIV; Non-Hodgkin lymphoma
| Introduction | ▴Top |
Plasmablastic lymphoma (PBL) is a rare, aggressive type of mature B-cell non-Hodgkin lymphoma with distinct morphological, immunophenotypical, and clinical features. PBL was first described as a unique entity arising from the oral cavity of patients with underlying human immunodeficiency virus (HIV) infection but has later been identified in patients with other immunodeficiencies and even in immunocompetent individuals [1, 2]. Immunophenotypically, PBL resembles late B cells that express plasma cell markers such as CD138, CD38, and MUM-1, instead of B-cell differentiation markers typical of diffuse large B-cell lymphoma (DLBCL), and thus is usually negative for CD20, CD79a and PAX5 [3]. The Ki-67 proliferation indices of these tumors are often high, reflecting their aggressive biology, and Epstein-Barr virus (EBV) is frequently detected in the majority of cases [1, 2].
The prognosis of PBL is historically poor, with median overall survival (OS) rates of 4 - 18 months [1, 4]. Factors such as poor performance status, advanced stage, and MYC aberrations have been correlated with poor prognosis, while EBV positivity and underlying HIV were reported to confer improved outcomes [1, 2, 4, 5].
Presently, limited literature pertaining to the prognosis or clinical management of patients presenting with PBL has resulted in no standard of care being established for patients with PBL [2, 6]. Due to the rarity of PBL, there remains a paucity of data specific to Asian populations. Current literature surrounding the epidemiology, clinical features, and outcomes of PBL in Asian populations are largely constrained to isolated reports and case series.
We were given the opportunity to conduct a retrospective chart review of 10 patients diagnosed with PBL, specifically examining their treatment outcomes and prognosis, highlighting features of PBL unique to an Asian demographic.
| Materials and Methods | ▴Top |
Study cohort
Ten patients who were diagnosed with PBL and seen at the National Cancer Centre Singapore between 2008 and 2019 were retrospectively analyzed. The median follow-up duration was 19.4 months.
Relevant demographical and clinicopathological information were collected and utilized for the analysis. Evaluation of complete response (CR) or partial response (PR) was conducted in accordance with the Cheson Tumor Response Assessment Criteria for malignant lymphomas [7]. Finally, pathological information such as tumor grade, tumor stage, number of lymph nodes involved, number of extranodal sites involved, and various immunohistochemical markers were included. EBV was identified via the detection of EBV-encoded RNA (EBER) on in-situ hybridization.
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. The research study was carried out with approval from the SingHealth Centralised Institutional Review Board (CIRB 2018/3084).
Literature search strategy
We performed a literature search on the PubMed MEDLINE database and the Cochrane Library to identify articles that were descriptive of similar studies of PBL in Asian cohorts. Case series and literature reviews were considered and reviewed, and relevant bibliographies of the retrieved studies were also screened to identify relevant data for inclusion. No time filters were applied during our search of the literature. The table highlighting the search terms used and the articles retrieved within each stage of the review are summarized in a PRISMA flow diagram (Supplementary Material 1, www.thejh.org). Articles were filtered out and omitted during screening if they did not fulfil certain requirements as follows: 1) Either a case series or literature review; 2) Primarily about an investigation of PBL; and 3) No reference to the same scientific study that a previous article has written on.
Statistical analysis
The outcomes of interest in this study are OS and progression-free survival (PFS). OS was calculated from the date of diagnosis up to the date of death from any cause or was censored at the date of last follow-up for survivors. PFS was defined as the time elapsed between the date of diagnosis to the date of relapse, progression, or death from any cause, whichever occurred first. Kaplan-Meier survival curves were plotted to estimate survival. Continuous variables and their associations were evaluated by Mann-Whitney U tests. All statistical evaluations were made assuming a two-sided test with significance level of 0.05. All tests were performed using MedCalc statistical software for Windows version 17.9 (MedCalc Software, Ostend, Belgium).
| Results | ▴Top |
Patient demographics and clinicopathological characteristics
The clinical characteristics and treatment outcomes of the 10 patients are summarized in Table 1 [8], and their hematological, biochemical, and histopathological profiles are summarized in Table 2. Of the 10 cases, nine (90%) were male, with median age at diagnosis of 55 years (range, 33 - 91 years). Seven (70%) patients were considered to be immunocompromised (five HIV/AIDS, two renal transplants). Patients with HIV/AIDS presented with a younger age of onset compared to others (median, 43 vs. 61 years; P = 0.0278). Median CD4 cell count at presentation for patients with HIV/AIDS was 68 per mm3 (range, 7 - 153). Two patients (cases 3 and 7) had pre-existing diagnosis of HIV/AIDS a year before lymphoma onset and were compliant to highly active antiretroviral therapy (HAART). Three patients (cases 4, 6, and 9) were diagnosed concurrently with lymphoma and commenced on HAART. Nine (90%) patients presented with an Eastern Cooperative Oncology Group (ECOG) status of 0 - 1, while only one (10%) patient presented with an ECOG status of 2. Six (60%) patients presented with early Ann Arbor stage disease (I and II), and four (40%) had advanced disease (III and IV). These observations point towards generally good functional status at diagnosis, despite several patients harbouring extensive disease and pre-existing immunodeficiency.
 Click to view | Table 1. Clinical Characteristics and Treatment Outcomes of Patients With Plasmablastic Lymphoma |
 Click to view | Table 2. Hematological, Biochemical and Histopathological Profiles |
A majority of patients in our case series presented with multiple sites of nodal involvement (median 1.5), especially in patients with pre-existing HIV/AIDS (median, 6 vs. 0; P = 0.0333). Mediastinal and axillary lymph nodes were most commonly involved (n = 5). Other common sites were retroperitoneal, iliac, supraclavicular, cervical, and hilar lymph nodes. Most patients (n = 9) had extranodal disease at diagnosis. The liver and posterior nasal space were the commonest sites involved, each presenting in two patients (20%).
From hematological profiles of the 10 patients, we identified eight (80%) with elevated serum lactate dehydrogenase (LDH) levels. All 10 (100%) patients presented with low serum albumin levels. Anemia was identified in five of 10 (50%) patients, and leucocytosis was identified in four of 10 (40%) of the patients. International prognostic index (IPI) scores were low (n = 5), low-intermediate (n = 2), high-intermediate (n = 2), and high (n = 1) [8]. IPI score on diagnosis was found to be positively correlated to HIV positivity (Cochran-Armitage test for trend, P = 0.034). Elevated LDH, anemia, leucocytosis, Ki67, OS and PFS had no significant relationship to HIV status.
Immunophenotypic characteristics
Molecular studies revealed a pattern of expression in keeping with known cases of PBL. All 10 patients were positive for one or more plasma cell markers CD38, CD138, and/or MUM-1 while expression of CD20 was universally absent, as is classically described. Surprisingly, EBER was positive in all patients, indicating a strong association with EBV in our cohort, which is higher than that reported in European-based studies of around 64-66% [1, 2]. CD79a staining was positive for four of eight (50%) cases tested; CD30 staining was positive in four of eight (50%) cases. The Ki-67 proliferation index was generally high (median, 80-90%), consistent with the highly aggressive nature of these tumors.
First-line treatment modalities and clinical outcomes
In the overall cohort, the median OS was 19.4 months. Five-year survival estimates were given at 60% and 36% for OS and PFS, respectively (Fig. 1a). Nine patients received initial treatment with combination chemotherapy (n = 8) or radiation alone (n = 1). One patient received best supportive care only. All treated patients achieved objective responses, including six (67%) with CRs and three (33%) with PRs. Among the eight patients that received chemotherapy, six were started on etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin (EPOCH)-based regimens, with or without consolidation radiotherapy. Remarkably, we observed that five out of these six patients (83%) achieved CR and one patient achieved PR. In this subgroup of patients treated with EPOCH-based regimens, the median PFS was 14.5 months and OS was 50.7 months. Of the two remaining cases, one patient received cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP), and the other received bortezomib, cyclophosphamide, vincristine, prednisone (B-CVP). Both achieved PR to their respective treatment regimens, with a PFS of 6.3 and 6.7 months respectively. Interestingly, one patient presenting with stage-IE PBL of the posterior nasal space had a durable CR to radiotherapy alone. Apart from case 6, all patients with HIV/AIDS achieved immune reconstitution with HAART after completion of chemotherapy. The clinical courses of all patients are summarized in Figure 1b.
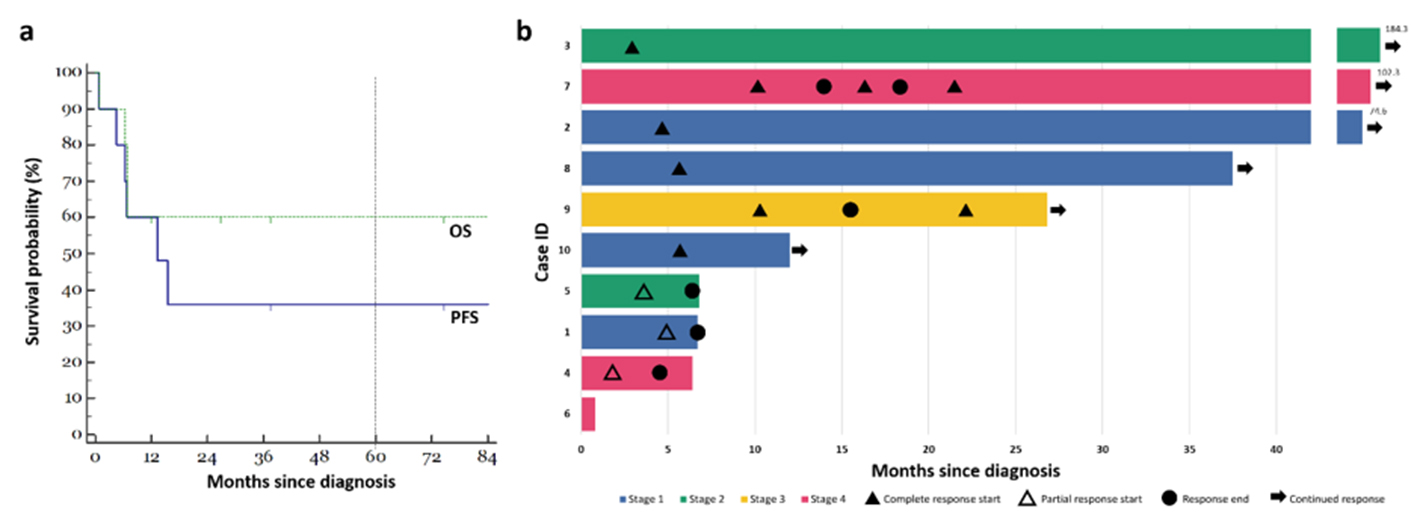 Click for large image | Figure 1. Clinical course of patients with plasmablastic lymphoma. (a) Kaplan-Meier estimates for our cohort of 10 patients. The green line indicates OS, while the blue line indicates PFS. Five-year absolute survival estimates were given at 60% and 36% for OS and PFS respectively. (b) Swimmer plot illustrating a summary of the clinical courses taken by each of the 10 patients in our cohort. OS: overall survival; PFS: progression-free survival. |
Imaging features of PBL
The clinical course and imaging features of case 7 are summarized in Figure 2. The patient had presented with multiple solid cystic masses within the abdomen, which achieved CR following bortezomib plus EPOCH (B-EPOCH) chemotherapy. However, the patient developed leptomeningeal relapse 4 months later. Resolution of disease was observed following methotrexate, procarbazine, vincristine (MPV) as second-line therapy.
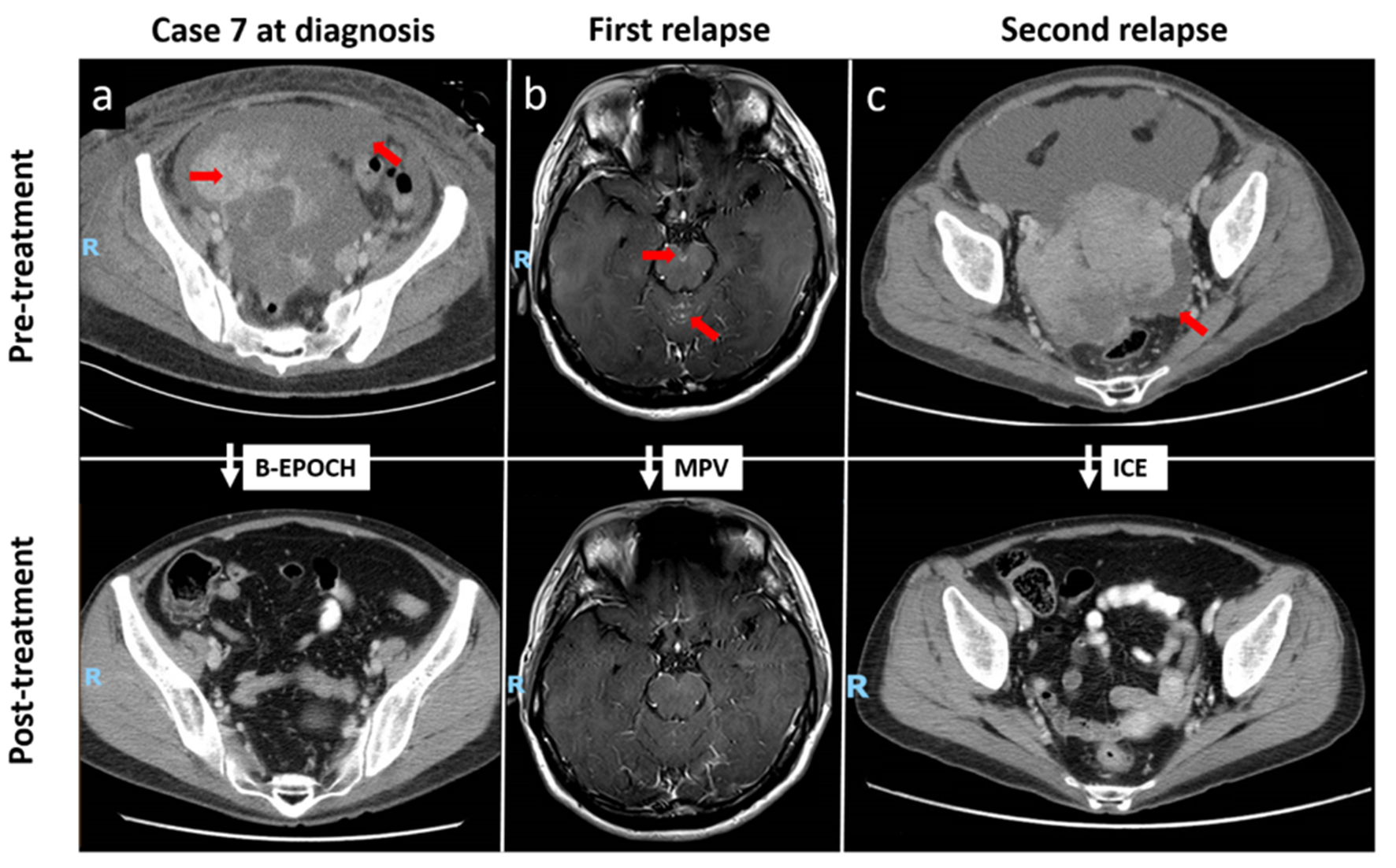 Click for large image | Figure 2. Repeated CRs to chemotherapy in a patient with relapsed stage IV plasmablastic lymphoma (case 7). (a) Contrast-enhanced CT illustrating CR of multiple solid cystic masses and peritoneal nodules following B-EPOCH as first-line therapy. (b) Contrast-enhanced T1-weighted MRI showing abnormal leptomeningeal enhancement at the cerebellar vermis and on the surface of the midbrain at level of the interpeduncular cistern, correlating with the presence of malignant blast cells on cerebrospinal fluid examination. Resolution of disease was observed following MPV as second-line therapy. (c) CR of enhancing soft-tissue masses in the pelvic cavity following third-line chemotherapy with the ICE regimen. Red arrows indicate initial sites of disease. CRs: complete responses; CT: computed tomography; B-EPOCH: bortezomib plus etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin; MRI: magnetic resonance imaging; MPV: methotrexate, procarbazine, vincristine; ICE: ifosfamide, carboplatin, etoposide. |
Two months later, the patient relapsed again with soft-tissue masses in the pelvic cavity, which again yielded a CR following ifosfamide, carboplatin, etoposide (ICE) chemotherapy. Despite multiple relapses, the patient has been in remission for the past 81 months.
18-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) imaging was also performed on selected patients. The patients presented with disease of the oral cavity (case 1), anorectum (case 9) and chest wall (case 10). At diagnosis, all three patients had primary tumors with high baseline metabolic activity (SUVmax 21.5, 30.9, and 28.2, respectively). End of treatment FDG-PET/CT images were available for cases 9 and 10, both after EPOCH chemotherapy followed by consolidation radiotherapy. Both patients achieved complete metabolic remission (SUVmax 9.4 and 4.6, respectively). Case 1 achieved PR after one cycle of B-CVP but died of pneumonia shortly after (Fig. 3).
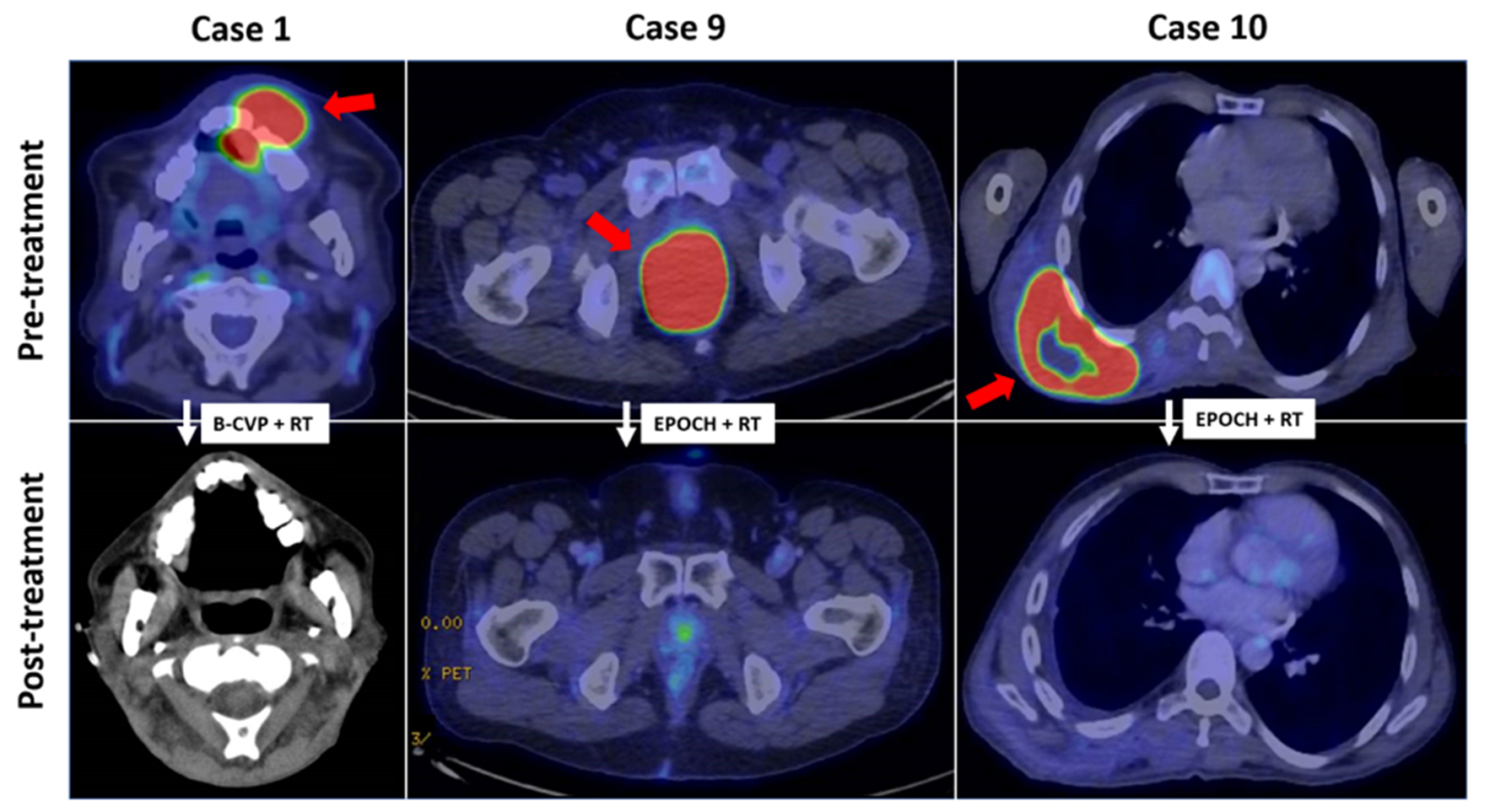 Click for large image | Figure 3. FDG-PET/CT imaging features in plasmablastic lymphoma. (case 1) FDG-avid soft tissue mass (SUVmax 21.5) in the oral cavity in partial response following B-CVP and consolidation radiotherapy. (case 9) Locally-advanced FDG-avid anorectal mass (SUVmax 30.9) in complete metabolic remission after EPOCH and consolidation radiotherapy. (case 10) Complete metabolic response of initially FDG-acid posterior chest wall mass (SUVmax 28.2) after EPOCH and radiotherapy. Red arrows indicate initial sites of disease. FDG-PET/CT: 18-fluorodeoxyglucose positron emission tomography/computed tomography; B-CVP: bortezomib, cyclophosphamide, vincristine, prednisone; EPOCH: etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin. |
| Discussion | ▴Top |
Our cohort presents with a clinicopathological landscape representative of Asian PBL. Like previous reports, most of our patients were immunocompromised (70%), with five out of the seven (71%) presenting with pre-existing HIV/AIDS [1]. As expected, the survival outcomes of patients with PBL in our cohort were dismal, with a median OS of 19.4 months despite most of them receiving multiagent systemic chemotherapy [1, 2]. Nonetheless, we observed that selected patients were able to achieve durable CRs to treatment (cases 2 and 3), even in the setting of advanced stage, high-risk IPI score and underlying immunodeficiency (case 7). In particular, case 7 was treated with B-EPOCH chemotherapy, a promising regimen which has recently been suggested to be highly effective in this disease [9, 10]. On another note, an elderly patient (case 8) with localized PBL of the posterior nasal space was able to achieve long-term remission with radiation therapy alone.
The immunophenotypic characteristics of PBL in our cohort are in keeping with that of classical PBL, expressing CD38, CD138, and MUM-1 amidst a negativity of pan-B cell markers such as CD20, along with high Ki-67 proliferation indices (median 80-90%) [1, 2]. As previously reported in other Asian cohorts and summarized in the results of our literature review (Table 3), HIV and EBV are both present in a significant proportion of our patients with PBL [11-18]. Similar to previous studies, these patients with HIV-associated PBL tended to be of younger age, and present with more advanced disease and higher IPI scores when compared to HIV-negative cases [4, 5]. Notably, the median CD4 cell count at presentation was 68 per mm3, which was lower than that previously reported by Castillo et al at 206 cells per mm3, suggesting that a greater degree of immunocompromise exists in our patients [4]. Yet despite these adverse features, most of the patients tolerated EPOCH-based chemotherapy and all achieved objective responses, including three of four in CR. This observation is in line with previous reports of HIV-related PBL achieving superior responses to chemotherapy and improved prognoses, possibly as a consequence of restored immunosurveillance through the use of HAART [5]. Taken together, PBL arising in HIV-positive and HIV-negative individuals harbours distinct clinical and pathological features, a finding consistent in both in Asian and Western cohorts.
 Click to view | Table 3. Glossary of Asian Studies of Plasmablastic Lymphoma |
FDG-PET/CT is currently the preferred imaging modality for staging and response assessment in FDG-avid lymphoma subtypes, including DLBCL and Hodgkin lymphoma [19]. Three of our patients who were staged with PET/CT included one with underlying HIV, a renal transplant recipient, and an immunocompetent individual. All of them harboured FDG-avid tumors, two of whom went into complete metabolic response after primary treatment, affirming the usefulness of PET/CT as a key modality for initial staging and response assessment in PBL [20].
In conclusion, our study describes the unique features of PBL in an Asian cohort and highlights the heterogeneity of clinical presentation, diversity of etiology, and disparate survival outcomes in this rare disease. Further research is warranted in this area of unmet clinical need.
| Supplementary Material | ▴Top |
Suppl 1. Search Terms Used for the Literature Search and PRISMA Flowchart of the Number of Studies Evaluated at Each Stage of the Systematic Review. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Acknowledgments
We would like to thank all subjects who have participated in this study.
Financial Disclosure
This work was supported by the Singapore Ministry of Health’s National Medical Research Council of Singapore (TCR12DEC005, NMRC/FLWSHP/054/2017-00), Tanoto Foundation Professorship in Medical Oncology, New Century Foundation Limited, Ling Foundation, Singapore National Cancer Centre Research Fund, Biomedical Research Council of A*STAR, SHF-Foundation as well as the SingHealth Duke-NUS Academic Medical Centre and Oncology ACP.
Conflict of Interest
None to declare.
Informed Consent
Participants and/or their legal guardians provided informed consent for their data to be used in this research.
Author Contributions
DY, GFT and JYC analyzed the data and drafted the manuscript; EWYC, VSY, EP, NS, MF, TT, MT and STL contributed to patient data; DY and JYC designed the study, interpreted the results, and revised the manuscript; and all authors read and approved the final version of the manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Morscio J, Dierickx D, Nijs J, Verhoef G, Bittoun E, Vanoeteren X, Wlodarska I, et al. Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases. Am J Surg Pathol. 2014;38(7):875-886.
doi pubmed - Tchernonog E, Faurie P, Coppo P, Monjanel H, Bonnet A, Algarte Genin M, Mercier M, et al. Clinical characteristics and prognostic factors of plasmablastic lymphoma patients: analysis of 135 patients from the LYSA group. Ann Oncol. 2017;28(4):843-848.
doi pubmed - Lopez A, Abrisqueta P. Plasmablastic lymphoma: current perspectives. Blood Lymphat Cancer. 2018;8:63-70.
doi pubmed - Castillo JJ, Furman M, Beltran BE, Bibas M, Bower M, Chen W, Diez-Martin JL, et al. Human immunodeficiency virus-associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy. Cancer. 2012;118(21):5270-5277.
doi pubmed - Castillo JJ, Winer ES, Stachurski D, Perez K, Jabbour M, Milani C, Colvin G, et al. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk Lymphoma. 2010;51(11):2047-2053.
doi pubmed - Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125(15):2323-2330.
doi pubmed - Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586.
doi pubmed - International non-Hodgkin's lymphoma prognostic factors project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329(14):987-994.
doi pubmed - Castillo JJ, Guerrero-Garcia T, Baldini F, Tchernonog E, Cartron G, Ninkovic S, Cwynarski K, et al. Bortezomib plus EPOCH is effective as frontline treatment in patients with plasmablastic lymphoma. Br J Haematol. 2019;184(4):679-682.
doi pubmed - Dittus C, Grover N, Ellsworth S, Tan X, Park SI. Bortezomib in combination with dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) induces long-term survival in patients with plasmablastic lymphoma: a retrospective analysis. Leuk Lymphoma. 2018;59(9):2121-2127.
doi pubmed - Han X, Duan M, Hu L, Zhou D, Zhang W. Plasmablastic lymphoma: Review of 60 Chinese cases and prognosis analysis. Medicine (Baltimore). 2017;96(9):e5981.
doi pubmed - Miao L, Guo N, Feng Y, Rao H, Wang F, Huang Q, Huang Y. High incidence of MYC rearrangement in human immunodeficiency virus-positive plasmablastic lymphoma. Histopathology. 2020;76(2):201-211.
doi pubmed - Wang D, Zheng Y, Zeng D, Yang Y, Zhang X, Feng Y, Lu H. Clinicopathologic characteristics of HIV/AIDS-related plasmablastic lymphoma. Int J STD AIDS. 2017;28(4):380-388.
doi pubmed - Rudresha AH, Lakshmaiah KC, Agarwal A, Babu KG, Loknatha D, Jacob LA, Babu S, et al. Plasmablastic lymphoma in immunocompetent and in immunocompromised patients: Experience at a regional cancer centre in India. South Asian J Cancer. 2017;6(2):69-71.
- Chen BJ, Wang RC, Ho CH, Yuan CT, Huang WT, Yang SF, Hsieh PP, et al. Primary effusion lymphoma in Taiwan shows two distinctive clinicopathological subtypes with rare human immunodeficiency virus association. Histopathology. 2018;72(6):930-944.
doi pubmed - Liu F, Asano N, Tatematsu A, Oyama T, Kitamura K, Suzuki K, Yamamoto K, et al. Plasmablastic lymphoma of the elderly: a clinicopathological comparison with age-related Epstein-Barr virus-associated B cell lymphoproliferative disorder. Histopathology. 2012;61(6):1183-1197.
doi pubmed - Suzuki Y, Yoshida T, Nakamura N, Kamata H, Kotani S, Ohsaka M, Kajita S, et al. CD3- and CD4-positive plasmablastic lymphoma: a literature review of Japanese plasmablastic lymphoma cases. Intern Med. 2010;49(16):1801-1805.
doi pubmed - Kim JE, Kim YA, Kim WY, Kim CW, Ko YH, Lee GK, Choi SJ, et al. Human immunodeficiency virus-negative plasmablastic lymphoma in Korea. Leuk Lymphoma. 2009;50(4):582-587.
doi pubmed - Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068.
doi pubmed - Al Tabaa Y, Tchernonog E, Faurie P, Cottereau AS, Monjanel H, Bonnet A, Le Gouill S, et al. Post-treatment positron emission tomography-computed tomography is highly predictive of outcome in Plasmablastic lymphoma. Eur J Nucl Med Mol Imaging. 2018;45(10):1705-1709.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


