| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 10, Number 2, April 2021, pages 71-75
Severe Cystic Echinococcosis-Associated Immune Thrombocytopenic Purpura: A Case Report
Marc Bienza, Sami Morin-Ben Abdallaha, Christina Greenawayb, Jean Sebastien Pelletierc, Stephen Caplana, Hans Knechta, d
aDivision of Hematology, Department of Medicine, Jewish General Hospital, McGill University, Montreal, QC H3T 1E2, Canada
bDivision of Infectious Diseases, Department of Medicine, Jewish General Hospital, McGill University, Montreal, QC H3T 1E2, Canada
cDivision of General Surgery, Department of Surgery, Jewish General Hospital, McGill University, Montreal, QC H3T 1E2, Canada
dCorresponding Author: Hans Knecht, Division of Hematology, Department of Medicine, Jewish General Hospital, McGill University, Montreal, QC H3T 1E2, Canada
Manuscript submitted February 2, 2021, accepted February 18, 2021, published online April 27, 2021
Short title: Wet Purpura in Cystic Echinococcosis
doi: https://doi.org/10.14740/jh789
| Abstract | ▴Top |
We present a case of immune thrombocytopenic purpura (ITP), which leads to the diagnosis of severe cystic echinococcosis. Our patient presented with platelets of 5 × 109/L, and investigations uncovered multiple large echinococcal hepatic cysts, the largest of which measured 19.4 × 15 × 12 cm, and peritoneal implants. While initially refractory to prednisone and immunoglobulins, the ITP responded to dexamethasone. The echinococcosis was treated with albendazole followed by surgical resection of all lesions. Our patient’s disease course has evolved favorable since his initial treatment with an isolated episode of recurrent thrombocytopenia 2 years later, and has remained in remission for the past 2 years. While a causal association between echinococcosis and ITP cannot be confirmed, this case is a reminder of the importance of remaining inquisitive for atypical potential triggers of ITP. We also present a review of the limited literature on the association of parasitic infections and ITP.
Keywords: Immune thrombocytopenic purpura; Echinococcus; Hydatid cysts; Parasite; Thrombocytopenia
| Introduction | ▴Top |
Immune thrombocytopenic purpura (ITP) is an immune-mediated phenomenon of platelet destruction leading to profound thrombocytopenia and consequently a defective primary hemostasis of variable degree. While primary ITP is, by definition, not associated with a predisposing condition, secondary ITP is triggered by an underlying disease. Of those, parasitic infections are not established culprits of ITP. We however present and discuss a first case of ITP in the context for severe cystic echinococcosis.
| Case Report | ▴Top |
Our patient was born in Algeria and immigrated to North America at the age of 40 years. He was diagnosed with mild hydatid disease at the age of 47 years upon travelling back to Algeria, which remained untreated. He was otherwise healthy and took no medication.
At the age of 59 years, he presented to our emergency department with a 3-day history of gradual onset of petechiae and bruising followed by gingival bleed and epistaxis. A complete review of systems was otherwise unremarkable apart from subjective fevers and chills. Physical examination revealed normal vital signs and was significant for abdominal distension, a palpable intra-abdominal mass in the right and left hypochondrium and wet purpura over the buccal mucosa and petechiae over the limbs and trunk. Initial investigation showed a platelet count of 5 × 109/L with a normal white blood cell differential and hemoglobin (Table 1). Creatinine and liver function tests were normal. The blood smear confirmed thrombocytopenia with giant platelets and no platelet clumping. White blood and red blood cell morphology was otherwise normal. Hepatitis B showed immunization from previous exposure, while hepatitis C and human immunodeficiency virus (HIV) serologies were negative. A bone marrow aspirate and biopsy were deemed unnecessary. Our patient was diagnosed with ITP and was given intravenous immunoglobulins at a dose of 1 g/kg daily for 2 days and prednisone 100 mg orally twice daily × 7 days. Given the absence of response by day 8, prednisone was replaced by dexamethasone 40 mg orally once a day for 4 days then stopped. On day 10 of treatment, platelet count rose to 36 × 109/L and had normalized to 213 × 109/L by day 20 (Fig. 1).
 Click to view | Table 1. Blood Work at Presentation |
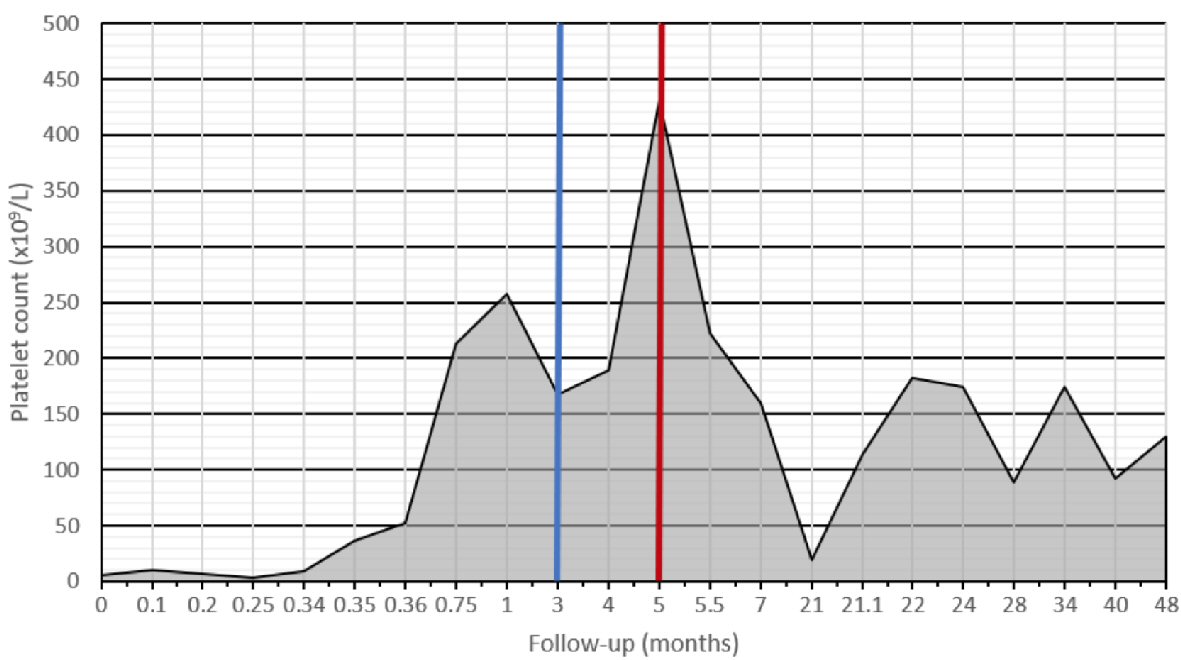 Click for large image | Figure 1. Platelet levels since diagnosis. Diagnosis was made at day 0. Albendazole was started at 3 months (blue line). The peak in platelet count at 5 months coincided with surgery (red line) and its associated inflammatory response. There was a period of loss to follow-up between 7 and 21 months after diagnosis. At 21 months, our patient had its first and only relapse. |
Bedside ultrasound performed to investigate the abdominal distension revealed a large abdominal cystic mass. A computed tomography (CT) scan showed multiloculated hepatic cysts contained in an inferior left lobe lesion measuring 19.4 × 15 × 12 cm extending anteriorly into the abdominal fat and the proximal transverse colon. A smaller 5.8 × 6.9 cm posterior hepatic lesion was also seen. Imaging findings were consistent with echinococcal hydatid disease (Fig. 2). There was no splenomegaly. The patient was referred to the Departments of Infectious Diseases and General Surgery. Albendazole was started 3 months later. After 1 month of treatment, a CT scan with contrast was performed and showed similar findings with the addition of an anterior peritoneal extrahepatic mass that had enlarged to 1.7 × 1.4 cm suggesting active echinococcal disease. A few weeks later, given concerns for rupture and anaphylaxis, our patient underwent partial left hepatectomy to remove the anterior cyst, unroofing of the posterior cyst and removal of two hydatid omental implants (Fig. 3). Corticosteroids were not administered perioperatively. Both the large anterior cyst and the smaller omental cysts showed presence of daughter cysts which suggested viability. Pathology confirmed the diagnosis of echinococcal cysts. Postoperative convalescence was uneventful without signs of sepsis or anaphylaxis. Albendazole was continued for a total of 6 months. Given the high risk of recurrence, our patient underwent close radiological follow-up and there has since been no evidence of echinococcal disease.
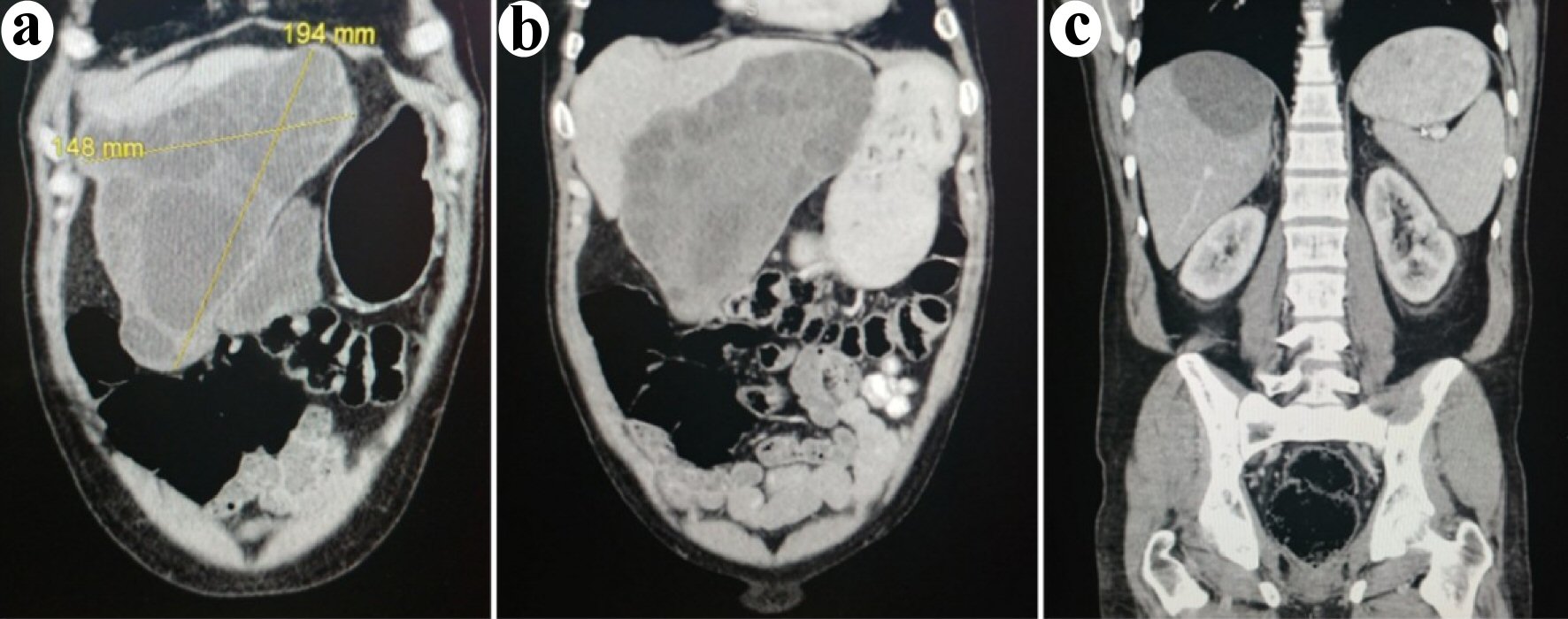 Click for large image | Figure 2. Coronal images of the computed tomodensitometry scan performed at presentation. (a) Anterior cut demonstrating the large left lobe cystic lesion extending downwards towards the proximal transverse colon. (b) Middle view of the abdomen demonstrating the same lesion. (c) Posterior view of the abdomen demonstrating the posterior liver lesion. |
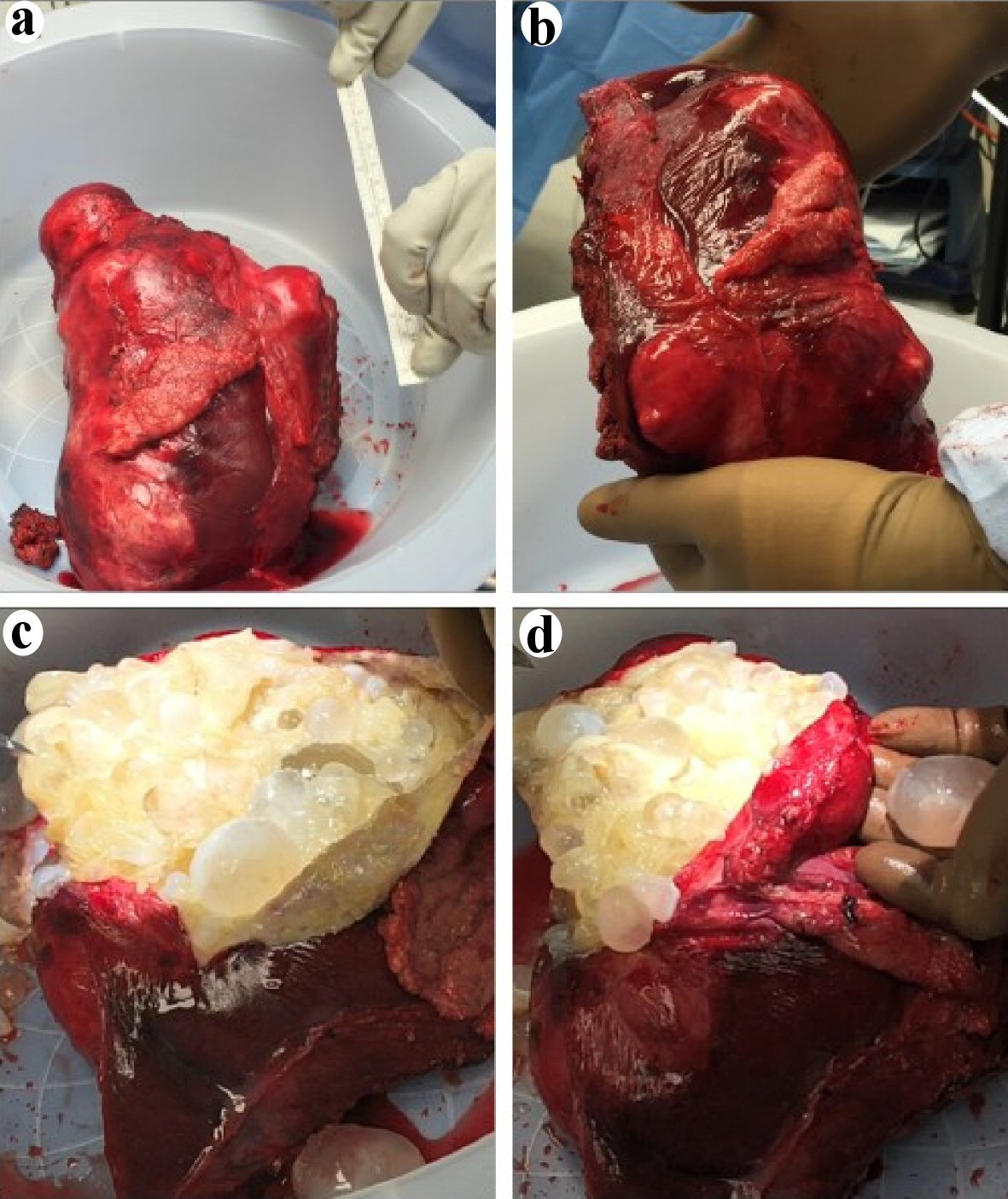 Click for large image | Figure 3. Postoperative partial hepatectomy specimen containing the large inferior left lobe hepatic cyst that was extending anteriorly into the abdominal fat and the proximal transverse colon. (a, b) View of the intact postoperative hepatic cyst and compared to a 15 cm (6 inches) ruler. (c, d) View of the inside of the large cyst following an excision of the cyst wall unveiling a multitude of intact smaller cysts of variable size containing a translucent fluid. |
Nearly 2 years after initial presentation with ITP, our patient relapsed and presented with a platelet count of 19 × 109/L. He responded well to dexamethasone 40 mg orally once daily for 4 days, and has remained in complete remission, without any further treatment with a total follow-up of 4 years since diagnosis (Fig. 1).
| Discussion | ▴Top |
A new diagnosis of ITP requires careful investigation for possible underlying predisposing conditions, the management of which is paramount to the successful treatment. Common causes of secondary ITP include viral infections, lymphoproliferative disorders, Helicobacter pylori and systemic lupus erythematosus among others (Table 2 [1, 2]). This case of ITP leads to the diagnosis of severe cystic echinococcosis, and albeit impossible to ascertain, prompted questioning on whether a causal effect was at play. This report gives an overview of the published evidence available associating parasitic infections and ITP.
 Click to view | Table 2. Causes of Secondary Immune Thrombocytopenic Purpura |
The impact on platelet function or number of many parasitic infections has been well documented, and a variety of pathogenic mechanisms have been hypothesized. In particular, the role of platelets in mediating infections from the Plasmodium species responsible for malaria has been extensively studied. Platelets have been shown to contribute to the innate immune response, have a direct cytotoxic effect and mediate cytotoxicity through platelet factor 4 against plasmodium [3]. In fact, animal studies showed that thrombocytopenic mice prior to infection had a worse outcome compared to those with normal platelet count. In humans thrombocytopenia is a poor prognostic marker [3]. Thrombocytopenia is a common finding in malaria and was described in 85% of Plasmodium falciparum and 72% of Plasmodium vivax malaria cases [4]. A retrospective study on 28 patients with malaria dating back to 1983 demonstrated that 17 of those had thrombocytopenia, of which 16 had positive platelet-associated immunoglobulin G (PaIgG). Both platelet count and PaIgG levels normalized after malaria treatment. Results from in vitro experiments suggest that immune-induced thrombocytopenia may occur as a result of the action of specific immunoglobulins binding to the H-malarial antigen bound to platelets through the Fab terminus [5]. Lacerda et al did in fact describe a case of ITP responsive to steroids in the context of a Plasmodium vivax malaria infection which relapsed with recurrence of the infection [6]. Yamaguchi et al also described two cases of thrombocytopenia associated with positive PaIgG and thought to be of immunological etiology. The platelet count and PaIgG levels improved after treatment of the malaria [7].
Cases of ITP in other parasitic infections are limited to case reports or animal studies. A case of babesiosis suspected to have triggered ITP in an otherwise healthy woman was described. Her ITP remained in complete remission following treatment of the parasitemia [8]. This clinical response resembles the favorable clinical course of our patient. A study in mice infected with Schistosoma mansoni has demonstrated the appearance of specific antibodies against mouse platelets and even human platelets causing a seemingly immune-dependent thrombocytopenia [9]. In a study on dogs with Leishmania infantum and Ehrlichia canis infections, platelet-bound antibodies were detected in 80% of co-infected dogs, 50% of leishmaniotic dogs and 60% of ehrlichiotic dogs, despite thrombocytopenia being a rare finding. According to the authors these results support the hypothesis of immune dysregulation of platelet function in these diseases often associated with hemorrhagic mortality [10].
Cystic echinococcosis is a zoonotic parasitic infection of which dogs are the definitive hosts and is caused by larvae of the Echinococcus granulosus tapeworms. Eggs gain access to circulation by hatching and penetrating the bowel wall and develop into metacestodes in affected organs leading to growth of large cysts [11]. While frequently asymptomatic, this disease can lead to organ failure and death. Surgical resection and anthelmintic medication such as albendazole are the mainstay of treatment of advanced disease [12]. Risks of the surgical resection include secondary echinococcosis from spillage of cyst content and anaphylaxis from exposure to high plasma concentration of echinococcal antigen [13]. Echinococcal disease has never been described as a cause of secondary ITP. Recent data have shown that ITP may be associated with complement activation [14], and that complement activating anti-Echinococcus specific antibodies may be found in echinococcosis [15]. There has however been no published case report or in vitro study on the association between thrombocytopenia and echinococcosis in the literature. In the case of our patient, PaIgG was not measured given their lack of sensitivity and specificity, and the diagnosis of ITP was based on the presence of profound thrombocytopenia of otherwise unclear cause and responsiveness to steroids.
In cases such as ours, other causes of thrombocytopenia must be investigated and ruled out. Splenomegaly is a common cause of thrombocytopenia in parasitic infections. Hepatosplenic Schistosomiasis mansoni has been shown to cause platelet pooling in the spleen of infected dogs [16]. Babesiosis, a tick-borne zoonotic parasite, has been associated with cytopenia and disseminated intravascular coagulation. Medication-induced thrombocytopenia must also be recognized, such as in the case of quinine for the treatment of malaria, which may induce immunologic thrombocytopenia or disseminated intravascular coagulopathy for the treatment of malaria [6, 17].
Whether the echinococcal infection in our otherwise healthy patient triggered the episode of ITP remains indeterminate, especially given the paucity of published data on non-malarial parasitic infections and ITP, and the fact the ITP relapsed 2 years later without evidence of recurrent echinococcosis. Nevertheless, this case is a reminder to the importance of remaining inquisitive for atypical potential triggers of ITP.
In conclusion, we present a case of ITP in the context of severe echinococcosis. Thrombocytopenia associated with parasitic infections must be thoroughly investigated, and a broad differential diagnosis should be considered which should include splenomegaly and platelet consumption. In cases where thrombocytopenia is severe and remains unexplained, the diagnosis of ITP could be entertained as a possible consequence from the parasitic infection. Albeit rare, ITP has been described in the literature in the context of some parasitic infections. In general, the pathogenesis of those cases remains elusive and more studies of such cases are warranted for further characterization. However, published data on the association between Plasmodium parasites and ITP has shed some light on some of the possible mechanisms of parasite-associated immune responses against platelets. In many cases of the literature as well as ours, management of both the parasitic infection and the ITP has led to favorable outcome.
Acknowledgments
The authors would like to thank the patient presented in this report for accepting and consenting to the publication of this manuscript.
Financial Disclosure
No funding was received. None of the authors have disclosures relevant to this manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
The manuscript has been sufficiently de-identified to protect the patient. Moreover, written consent was obtained.
Author Contributions
M. Bienz was responsible of the original draft preparation. S. Morin-Ben Abdallah was involved in initial treating and reviewing the manuscript. C. Greenaway, J-S Pelletier and S. Caplan were involved in the management of the case and reviewed the manuscript. H. Knecht is involved in the continued follow-up of the patient and supervised and reviewed the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within this article.
| References | ▴Top |
- Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, Ghanima W, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817.
doi pubmed - Consolini R, Costagliola G, Spatafora D. The centenary of immune thrombocytopenia-part 2: revising diagnostic and therapeutic approach. Front Pediatr. 2017;5:179.
doi pubmed - Alonso AL, Cox D. Platelet interactions with viruses and parasites. Platelets. 2015;26(4):317-323.
doi pubmed - Horstmann RD, Dietrich M, Bienzle U, Rasche H. Malaria-induced thrombocytopenia. Blut. 1981;42(3):157-164.
doi pubmed - Kelton JG, Keystone J, Moore J, Denomme G, Tozman E, Glynn M, Neame PB, et al. Immune-mediated thrombocytopenia of malaria. J Clin Invest. 1983;71(4):832-836.
doi pubmed - Lacerda MV, Alexandre MA, Santos PD, Arcanjo AR, Alecrim WD, Alecrim MG. Idiopathic thrombocytopenic purpura due to vivax malaria in the Brazilian Amazon. Acta Trop. 2004;90(2):187-190.
doi pubmed - Yamaguchi S, Kubota T, Yamagishi T, Okamoto K, Izumi T, Takada M, Kanou S, et al. Severe thrombocytopenia suggesting immunological mechanisms in two cases of vivax malaria. Am J Hematol. 1997;56(3):183-186.
doi - Narurkar R, Mamorska-Dyga A, Agarwal A, Nelson JC, Liu D. Babesiosis-associated immune thrombocytopenia. Stem Cell Investig. 2017;4:1.
doi pubmed - Stanley RG, Ngaiza JR, Atieno E, Jell G, Francklow K, Jackson CL, Parry H, et al. Immune-dependent thrombocytopaenia in mice infected with Schistosoma mansoni. Parasitology. 2003;126(Pt 3):225-229.
doi pubmed - Cortese L, Terrazzano G, Piantedosi D, Sica M, Prisco M, Ruggiero G, Ciaramella P. Prevalence of anti-platelet antibodies in dogs naturally co-infected by Leishmania infantum and Ehrlichia canis. Vet J. 2011;188(1):118-121.
doi pubmed - Brehm K, Koziol U. Echinococcus-host interactions at cellular and molecular levels. Adv Parasitol. 2017;95:147-212.
doi pubmed - Brunetti E, Kern P, Vuitton DA, Writing Panel for the W-I. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114(1):1-16.
doi pubmed - Tuxun T, Zhang JH, Zhao JM, Tai QW, Abudurexti M, Ma HZ, Wen H. World review of laparoscopic treatment of liver cystic echinococcosis - 914 patients. Int J Infect Dis. 2014;24:43-50.
doi pubmed - Castelli R, Lambertenghi Delilliers G, Gidaro A, Cicardi M, Bergamaschini L. Complement activation in patients with immune thrombocytopenic purpura according to phases of disease course. Clin Exp Immunol. 2020;201(3):258-265.
doi pubmed - Li Z, Zhang C, Li L, Bi X, Li L, Yang S, Zhang N, et al. The local immune response during Echinococcus granulosus growth in a quantitative hepatic experimental model. Sci Rep. 2019;9(1):19612.
doi pubmed - Correia MC, Domingues AL, Lacerda HR, Santos EM, Machado CG, Hora V, Neves MA, et al. Platelet function and the von Willebrand factor antigen in the hepatosplenic form of schistosomiasis mansoni. Trans R Soc Trop Med Hyg. 2009;103(10):1053-1058.
doi pubmed - Nadeem MK, Hammerl N. Quinine induced disseminated intravascular coagulopathy. Australas Med J. 2011;4(9):488-489.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


