| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 10, Number 2, April 2021, pages 80-82
MYC-Positive Diffuse Large B-Cell Lymphoma in Leukemic Phase at Presentation: A Diagnostic and Therapeutic Challenge
Yavuz Memis Bilgina, c, Rob Castelb
aDepartment of Internal Medicine, Admiraal de Ruyter Hospital, Goes, the Netherlands
bDepartment of Clinical Chemistry, Admiraal de Ruyter Hospital, Goes, the Netherlands
cCorresponding Author: Yavuz Memis Bilgin, Department Internal Medicine, Admiraal de Ruyter Hospital, PO Box 15, 4460 AA Goes, the Netherlands
Manuscript submitted March 16, 2021, accepted March 30, 2021, published online April 27, 2021
Short title: DLBCL Presenting in Leukemic Phase
doi: https://doi.org/10.14740/jh818
| Abstract | ▴Top |
Diffuse large B-cell lymphoma (DLBCL) in leukemic phase at presentation is a rare condition, and it can be challenging to differentiate from acute leukemia or other types of non-Hodgkin lymphoma. To obtain an accurate diagnosis immunophenotyping and cytogenetic analyses should be performed. Herein, we report a 54-year-old woman who experienced loss of consciousness and fever. Laboratory test results revealed leukocytosis, anemia, thrombopenia and hypercalcemia. Morphology of blood smear revealed two abnormal cell populations. However a specific diagnosis could not be made. Immunophenotyping showed two different populations, which was consistent with non-Hodgkin lymphoma. A fluorescence in situ hybridization (FISH) showed MYC and BCL2 rearrangements. Finally a leukemic DLBCL was diagnosed and immediately treatment with rituximab cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) was started. Due to MYC-positivity, lenalidomide was added to the therapy regimen. After treatment the patient achieved complete remission without any clinical sequelae, which is still ongoing after 4 years. Lenalidomide is an oral immunomodulatory drug that downregulates MYC gene and is commonly used in patients with multiple myeloma. Moreover, it can also be a promising therapeutic option for patients with MYC-positivity DLBCL presenting in leukemic phase.
Keywords: DLBCL; Leukemic phase; Lenalidomide
| Introduction | ▴Top |
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphomas (NHLs), and it accounts about one-third of all types of NHL [1]. Its prognosis is based on the International Prognostic Index (IPI) score and genomic markers, and it is treated with anthracycline/rituximab-based chemotherapy. However DLBCL presenting in leukemic phase is extremely rare and can be challenging to differentiate from acute leukemia or other types of NHL [2-4]. Therefore, to obtain an accurate diagnosis immunophenotyping and cytogenetic analyses must be performed. Due to a high tumor burden the early mortality rate of leukemic DLBCL is high, however the overall survival is comparable to patients with DLBCL without leukemic presentation and a high IPI-scores. Herein, we present a patient who had a severe presentation of a leukemic DLBCL with both diagnostic and therapeutic challenges.
| Case Report | ▴Top |
A 54-year-old woman was admitted to the emergency room due to loss of consciousness and fever. The blood count examination revealed the following results: hemoglobin 7.1 g/dL (normal 12.1 - 15.1), leukocytes 52 × 109/L (normal: 4 - 10), platelets 79 × 109/L (normal: 150 - 400), calcium 5.13 mmol/L (normal: 2.10 - 2.55), lactate dehydrogenase (LDH) 1,740 U/L (normal: < 250).
The peripheral blood smear showed two abnormal cell populations: one population had large cells comprising 24% of the nucleated cells with an irregular cell outline, scant basophilic cytoplasm and irregular multiple distinct nucleoli with a diffuse chromatin pattern. These cells were probably of lymphoid origin; however the presence of leukemic blasts was not clear. The other comprised small lymphocytes with regular round nuclei, condensed chromatin pattern and scanty cytoplasm (Fig. 1). Moreover, the peripheral blood immunophenotyping showed also two different populations: one had 54% monoclonal B-cells which were positive for cluster of differentiation (CD)19, CD20, CD22, CD45, CD79a, CD10, CD38, and sIg lambda and negative for CD34, TdT, and cyclin D1. The other comprised 11% monoclonal B-cells which were positive for CD19, CD20, CD10, CD38, and negative for CD22 and sIgM. Acute leukemia or precursor lymphoblastic lymphoma was ruled according to the absence of CD34 and TdT. Further cyclin D1 negativity excluded a mantle cell lymphoma. The presence of both populations was consistent with NHL. The bone marrow was hypercellular with extensive infiltration of lymphoblastoid cells. The result of immunophenotyping was similar to that of the peripheral blood examination. The Ki-67 index was high (80%). Fluorescence in situ hybridization (FISH) revealed translocations of the MYC and BCL2 genes. The BCL6 gene was negative, thereby excluding Burkitt lymphoma. Finally, the patient was diagnosed with leukemic DLBCL. The immunohistochemical analysis was consistent with germinal center subtype and cytogenetic analysis revealed a double-hit lymphoma. According to the World Health Organization (WHO) 2017 classification, the diagnosis was high-grade B-cell lymphoma with MYC and BCL2 rearrangements. A positron emission tomography/computed tomography (PET/CT) scan showed uptake in the para-aortic and para-iliac lymph nodes, bone marrow and spleen. A Lumbar puncture showed no lymphoma localization in the cerebrospinal fluid. The disease was classified as stage IV based on the Ann Arbor staging system and the revised IPI-score was 4. The patient was admitted to the intensive care unit and underwent hemodialysis for hypercalcemia. After diagnosis, she immediately started treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). After the first cycle the patient immediately recovered and the laboratory results normalized. After confirmation of MYC positivity the treatment was continued and lenalidomide was added to the therapy, according to the HOVON-130 study protocol. She received six cycles of R-CHOP in combination with lenalidomide, followed by two single doses with rituximab. After treatment no clinical sequelae were observed and her normal blood count was normal. Both PET/CT scan and bone marrow biopsy showed complete remission, which is still ongoing for 4 years.
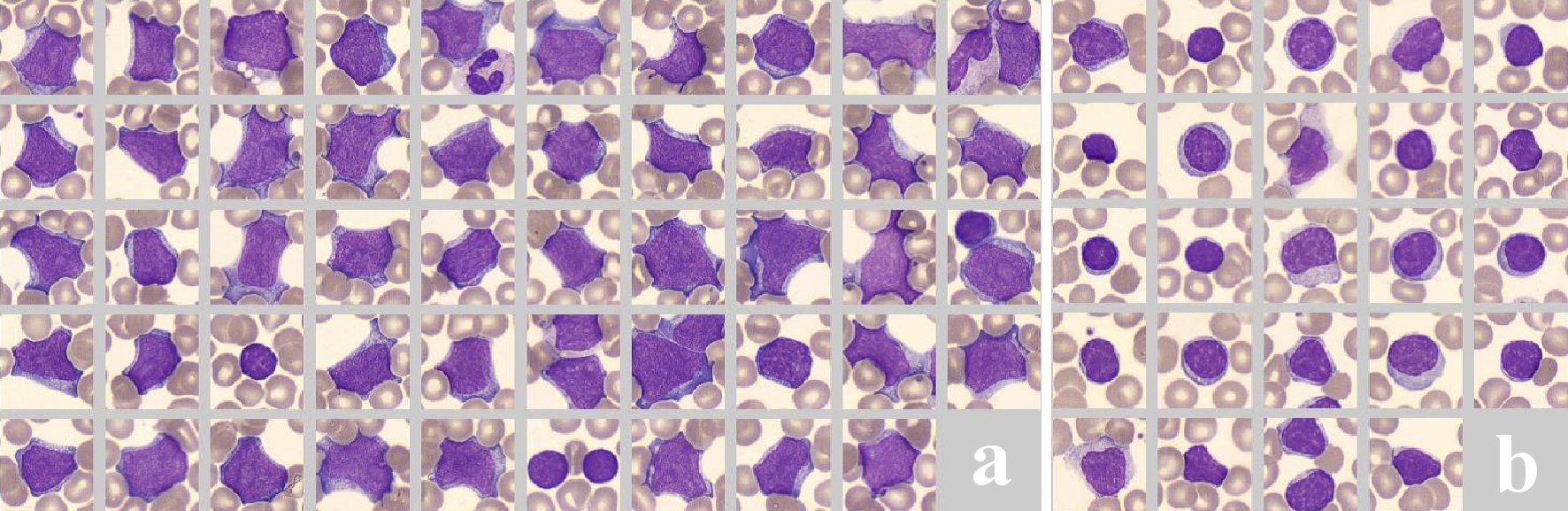 Click for large image | Figure 1. Peripheral blood smear showing two different cell populations. (a) One with large cells with an irregular cell outline, scant basophilic cytoplasm and irregular multiple distinct nucleoli with a diffuse chromatin pattern. (b) The other comprised small lymphocytes with regular round nuclei, condensed chromatin pattern and scanty cytoplasm. |
| Discussion | ▴Top |
DLBCL is the most common type of NHL. However, DLBCL presenting in leukemic phase is rare and commonly involves of extra-nodal sites such as bone marrow, spleen, lung, liver, bowels and cerebrospinal fluid. The presence of circulating lymphoma cells in the blood is often observed in mantle cell lymphoma, follicular lymphoma, and anaplastic large cell lymphoma. Further, it is observed in terminal phases of all refractory lymphomas. The mechanisms of lymphoma cell migration into the bloodstream are unclear [5]. To obtain accurate diagnosis, immunophenotyping and cytogenetic analysis must be performed. Hence, other types of lymphomas and leukemia can be ruled out. In our case the morphology was compatible with aggressive lymphomas or acute leukemia. However both were ruled out based on the immunophenotyping. Further the cytogenetic analysis was essential in obtaining an accurate diagnosis.
DLBCL presenting in leukemic phase has a high tumor burden. Moreover, it is associated with a high early mortality rate compared to patients diagnosed with DLBCL who received standard R-CHOP regimen (14% vs. 6%). However the 4-year survival in both patient groups is comparable (approximately 50%) [6]. Treatment with intensive chemotherapy or autologous stem cell transplantation does not improve overall survival and is associated with increased toxicity [7].
In the revised WHO 2017 classification leukemic DLBCL is not a separate entity. In addition, the importance of gene rearrangements has been emphasized. They should be investigated in all patients with newly diagnosed with DLBCL [8]. Our patient had MYC and BCL2 rearrangements, which are indicative of double-hit lymphoma. Moreover, according to the WHO 2017 classification it is considered as high-grade B-cell lymphoma with MYC and BCL2 rearrangements. High-grade lymphoma has been recently introduced for aggressive lymphomas, and it is similar to DLBCL in terms of clinical and morphologic features. In addition to the IPI-score, the outcome of lymphomas is depending on the gene rearrangements. MYC rearrangement is observed in 10-15% of patients with DLBCL, and it has a negative impact on prognosis [9]. The 5-year overall survival of MYC-positive patients treated with RCHOP was lower than that of patients with MYC-negative DLBCL (31% vs. 66%) [10]. Therefore, for this type of lymphomas more intensive chemotherapy regimens are recommended.
Lenalidomide is an oral immunomodulatory drug that downregulates MYC, and its target genes and is commonly administered to patients with multiple myeloma. Therefore, it is suitable for patients with MYC-positive lymphoma. The toxicity of lenalidomide is lower than that of intensive chemotherapy regimens. Recently, in the Netherlands, the HOVON-130 study was conducted on patients with MYC-positive high-grade lymphoma. They received R-CHOP combined with lenalidomide (15 mg for days 1 - 14) for six cycles, followed by two single doses with rituximab. The 2-year overall survival rate of these patients was 73% [11]. Our patient received this regimen 4 years ago. Since then she is still on complete remission.
Conclusions
DLBCL presenting in leukemic phase is a rare condition and it is difficult to differentiate from leukemia or other aggressive lymphomas. Immunophenotyping and cytogenetic analysis must be performed for an accurate diagnosis. In terms of treatment, lenalidomide combined with anthracycline/rituximab-based chemotherapy is a promising therapeutic option for patients with MYC-positivity DLBCL presenting in leukemic phase. In this case, both the diagnosis and treatment of DLBCL were challenging.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent for the publication of this report was obtained from the patient.
Author Contributions
YMB wrote the initial draft and participated in the clinical care. RC provided morphologic images. Both authors reviewed the final draft and approved its submission.
Data Availability
The authors declare that data supporting the findings of this study are available in the article.
| References | ▴Top |
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390.
doi pubmed - Bain BJ, Catovsky D. The leukaemic phase of non-Hodgkin's lymphoma. J Clin Pathol. 1995;48(3):189-193.
doi pubmed - Balasubramanian P, Ramteke P, Mallick S, Kumar L, Tanwar P. Diffuse large B-cell lymphoma relapsing in leukaemic phase presenting as acute leukaemia. Clin Med Insights Blood Disord. 2019;12:1179545X18821160.
doi pubmed - Suresh PK, Basavaiah SH, Goel M, Dsouza S, Rai S. Diffuse large B-cell lymphoma in blood and bone marrow: a rare presentation of leukemic phase at diagnosis. Hematol Transfus Cell Ther. 2020;42(2):180-183.
doi pubmed - Pires PP, Kanegae MY, Rays J, Catania M, Lima FR, Noronha TR, Abdo AN, et al. Diffuse large B-cell lymphoma presenting in the leukemic phase. Autops Case Rep. 2016;6(1):41-45.
doi pubmed - Muringampurath-John D, Jaye DL, Flowers CR, Saxe D, Chen Z, Lechowicz MJ, Weisenburger DD, et al. Characteristics and outcomes of diffuse large B-cell lymphoma presenting in leukaemic phase. Br J Haematol. 2012;158(5):608-614.
doi pubmed - Howlett C, Snedecor SJ, Landsburg DJ, Svoboda J, Chong EA, Schuster SJ, Nasta SD, et al. Front-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: a systematic review and meta-analysis. Br J Haematol. 2015;170(4):504-514.
doi pubmed - Scott DW, King RL, Staiger AM, Ben-Neriah S, Jiang A, Horn H, Mottok A, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060-2064.
doi pubmed - Rosenwald A, Bens S, Advani R, Barrans S, Copie-Bergman C, Elsensohn MH, Natkunam Y, et al. Prognostic significance of MYC rearrangement and translocation partner in diffuse large B-cell lymphoma: a study by the lunenburg lymphoma biomarker consortium. J Clin Oncol. 2019;37(35):3359-3368.
doi pubmed - Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, Horsman DE, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114(17):3533-3537.
doi pubmed - Chamuleau MED, Burggraaff CN, Nijland M, Bakunina K, Mous R, Lugtenburg PJ, Dierickx D, et al. Treatment of patients with MYC rearrangement positive large B-cell lymphoma with R-CHOP plus lenalidomide: results of a multicenter HOVON phase II trial. Haematologica. 2020;105(12):2805-2812.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


