| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Review
Volume 11, Number 5, October 2022, pages 159-166
Intravenous Fluid Administration and the Risk of Adverse Outcomes in Sickle Cell Disease Patients Hospitalized for Vaso-Occlusive Crisis
Ademola S. Ojoa, c , Somtochukwu Ojukwua, Wassihun Asmarea, Oluwamayowa Odipeb, Daniel Larbia
aDepartment of Internal Medicine, Howard University Hospital, Washington, DC, USA
bDepartment of Pediatrics and Child Health, Queen’s Medical Center, Nottingham University Hospitals NHS Trust, Nottingham, UK
cCorresponding Author: Ademola S. Ojo, Department of Internal Medicine, Howard University Hospital, Washington, DC, USA
Manuscript submitted September 21, 2022, accepted October 26, 2022, published online October 31, 2022
Short title: IVFs Treatment in SCD Patients With VOC
doi: https://doi.org/10.14740/jh1058
| Abstract | ▴Top |
Vaso-occlusive crisis (VOC) is the leading cause of hospitalization in sickle cell disease (SCD). Intravenous fluid (IVF) administration is the usual practice during VOC episodes to slow the sickling process. In the absence of an evidence-based, clear-cut consensus on the optimal choice, route, and rate of fluid administration, there has been a wide variability in the practice of IVF administration in the treatment of VOC. However, there are growing concerns about the safety of this practice. This systematic review summarized the current evidence on the risk of negative outcomes in SCD patients treated for VOC with IVFs. A database search of Medline/PubMed, EMBASE, Scopus, Web of Science, CINAHL, Wiley Cochrane Library, Clinicaltrials.gov, and conference proceedings of the European Hematology Association (EHA) and American Society of Hematology (ASH) were performed. The results were presented using narrative analysis of quantitative data. Of the 2,821 identified records, a total of three eligible retrospective cohort studies with a total demographic population of 549 SCD patients were included in this review. Normal saline, a frequently used IVF for VOC may be associated with adverse outcomes such as poor pain control and volume overload. Volume overload, new oxygen requirement, acute chest syndrome, and acute kidney injury are potential adverse outcomes of inappropriate IVF use in VOC. There is limited evidence supporting the current practice of IVF use in VOC. Randomized controlled trials are required to fully clarify the place and safety of IVF in the management of VOC.
Keywords: Sickle cell disease; Vaso-occlusive crisis; Intravenous fluid; Adverse outcome; Normal saline
| Introduction | ▴Top |
Sickle cell disease (SCD) spectrum is an umbrella term describing a group of monogenic disorders characterized by defects in the β hemoglobin subunit due to mutations in the hemoglobin beta (HBB) gene on chromosome 11 [1]. The underlying genetic abnormality in SCD is the inheritance of hemoglobin S allele in homozygosis or in heterozygosis with another defective beta hemoglobin gene. The disease spectrum includes individuals with homozygous disease (HbSS); the most severe variant, and other compound heterozygous forms such as HbSC, HbSOArab, HbSDPunjab, HbS/β0-thalassaemia, and HbS/β+-thalassemia [2]. Millions of people are affected by SCD globally and the disease is most commonly found among individuals of African, Caribbean, Indian, and Saudi Arabian ancestry, as well as in certain regions of the Mediterranean, Central, and South America [3]. The polymerization of deoxygenated hemoglobin is the central theme of SCD, leading to the sickling of red blood cells (RBCs), vaso-occlusion, hemolysis, and other systemic manifestations of SCD. Vaso-occlusive crisis (VOC) is the most common clinical manifestation of SCD and is the leading cause of emergency department (ED) visits and hospitalization [4]. In the United States which has an SCD population of about 100,000, data from the Healthcare Cost and Utilization Project (HCUP) reported over 100,500 hospitalizations for VOC in 2016, resulting in a total hospitalization cost of over $800 million [5]. This accounts for only about 40% of the total annual ED visits for VOC [6].
Traditionally, the treatment of VOC involves the administration of fluids and analgesics. This approach is based on the perceived fluid balance abnormalities in SCD. One of the earliest studies on fluid balance disorders in SCD evaluated 24 patients in a steady state and during a crisis and reported a persistent negative fluid balance (urine output > intake) regardless of fluid intake and an inability of the kidneys to concentrate urine during fluid restriction [7]. While this state of negative fluid balance may potentially play a role in VOC through reduced flow in the microcirculation, evidence shows intracellular dehydration is the main culprit in the development of VOC [8]. In sickle cells, cellular dehydration occurs as a result of water loss accompanied by loss of potassium and chloride ions from reticulocytes and matured RBCs due to alterations in membrane permeability resulting from changes in intracellular pH and calcium [9]. Dehydrated sickle RBCs have concentrated HbS, which sickles very easily [8]. Many studies have demonstrated the role of cellular HbS concentration in sickling, with a small rise in HbS concentration creating a significant increase in cell sickling [10-12]. However, there is insufficient evidence connecting the state of negative fluid balance in SCD with cellular dehydration and it is uncertain if intravenous fluid (IVF) administration has the potential to reverse the cellular dehydration. While Rosa et al 1980 demonstrated reduced sickling by causing cellular hydration and swelling by inducing hyponatremia, such an approach was not practicable in clinical practice [13]. In the absence of any other superior therapeutic intervention, fluid administration remained the core of VOC treatment.
The lack of sufficient evidence on fluid administration is further reflected in the lack of a consensus on the optimal fluid and route of administration for VOC [14]. Intravenous rather than oral fluid is usually the rule, and this is often administered as boluses and/or continuous infusions at a high rate [15]. The type of fluids used often varies from facility to facility, even within the same facility, variations exist in the types of fluids administered [16]. One study reported normal saline bolus as the most commonly administered fluid regimen by ED physicians in a facility despite institutional practice guideline recommendation of 5% dextrose with 1/4 normal saline (NS) [16]. However, the use of IVFs is not without complications and more so in SCD patients who often have varying degrees of renal, cardiac, and pulmonary dysfunction.
This study aims to investigate the safety of the current practice of IVF administration in the treatment of sickle cell VOC through a comprehensive literature search. We evaluated the risk of negative outcomes in SCD patients treated for VOC with IVFs. Evidence from this study will highlight the potential risks associated with the current practice of IVF use to reduce treatment-associated morbidity and improve outcomes in SCD patients hospitalized for VOC.
| Methods | ▴Top |
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) guideline was adopted for this study [17].
Search strategy
A comprehensive search of Medline/PubMed, EMBASE, Scopus, Web of Science, CINAHL, Wiley Cochrane Library, and Clinicaltrials.gov databases was performed to identify relevant articles from database inception till August 25, 2022. In addition, conference proceedings of the American Society of Hematology (ASH) and the European Hematology Association (EHA) were evaluated for relevant studies. The following search terms were used: “sickle cell AND vaso-occlusive crisis”, “sickle cell AND intravenous fluid”, “vaso-occlusive crisis AND intravenous fluid”, “fluid AND sickle cell”, “sickle cell AND hydration”, “saline AND sickle cell”. We expanded the search coverage by using the vague term “sickle cell” in the search strategy to identify more potentially relevant articles. The citations within the studies were assessed for other potentially suitable studies. An example of the search strategy is provided here (Supplementary Material 1, www.thejh.org).
Eligibility criteria
Type of studies
The studies included in this review were mainly retrospective studies that assessed the risk of adverse outcomes following the use of IVFs in the treatment of VOC.
Types of participants
All SCD patients who were hospitalized with a primary diagnosis of VOC.
Interventions
IVFs of any type administered as a bolus or continuous infusion.
Exclusion criteria
Studies with a quality score of 6 or less on the modified Newcastle-Ottawa scale or studies that lacked sufficient data on the above-mentioned domains were excluded. Commentaries, editorials, reviews, and case reports were excluded.
Study screening, selection, and data extraction
Potentially suitable studies were independently evaluated by two reviewers. Any conflicts were resolved through discussion and if indicated, through a third reviewer. The titles and abstracts of the search results were screened for eligibility followed by the removal of duplicate studies. All potentially suitable studies underwent full-text review to assess eligibility. Eligible studies were included in this review. Data were extracted using a pre-designed Excel sheet. The following information, when available was extracted from the studies: author, year of publication, type of study, sample size, type of IVF administered, the average volume of IVF administered, bolus vs. continuous fluid administration, and documented adverse outcomes.
Quality assessment and data synthesis
All included studies were critically appraised for methodological quality. Since all included studies were cohort studies, the Newcastle-Ottawa scale for assessing the quality of cohort studies in a systematic review was used [18]. The domains assessed include the selection of cohorts, comparability of cohorts, and assessment of outcomes (Supplementary Material 2, www.thejh.org). Data analysis was conducted using a narrative approach. A meta-analysis was considered inappropriate due to limited data and significant heterogeneity in the measurement of the outcomes evaluated.
| Results | ▴Top |
The summary of the study selection is displayed in Figure 1. A total of 2,821 records were identified through database search, conference proceedings, and a review of citations. The title and abstracts of 1,002 studies were reviewed for eligibility after the removal of duplications. Twenty-two potentially suitable studies underwent full-text review for eligibility. Nineteen studies were excluded based on the following reasons: interventions not relevant (15), adverse outcomes not specified (three), and duplicate patient population (one). The characteristics of the included studies are shown in Table 1 [19-21].
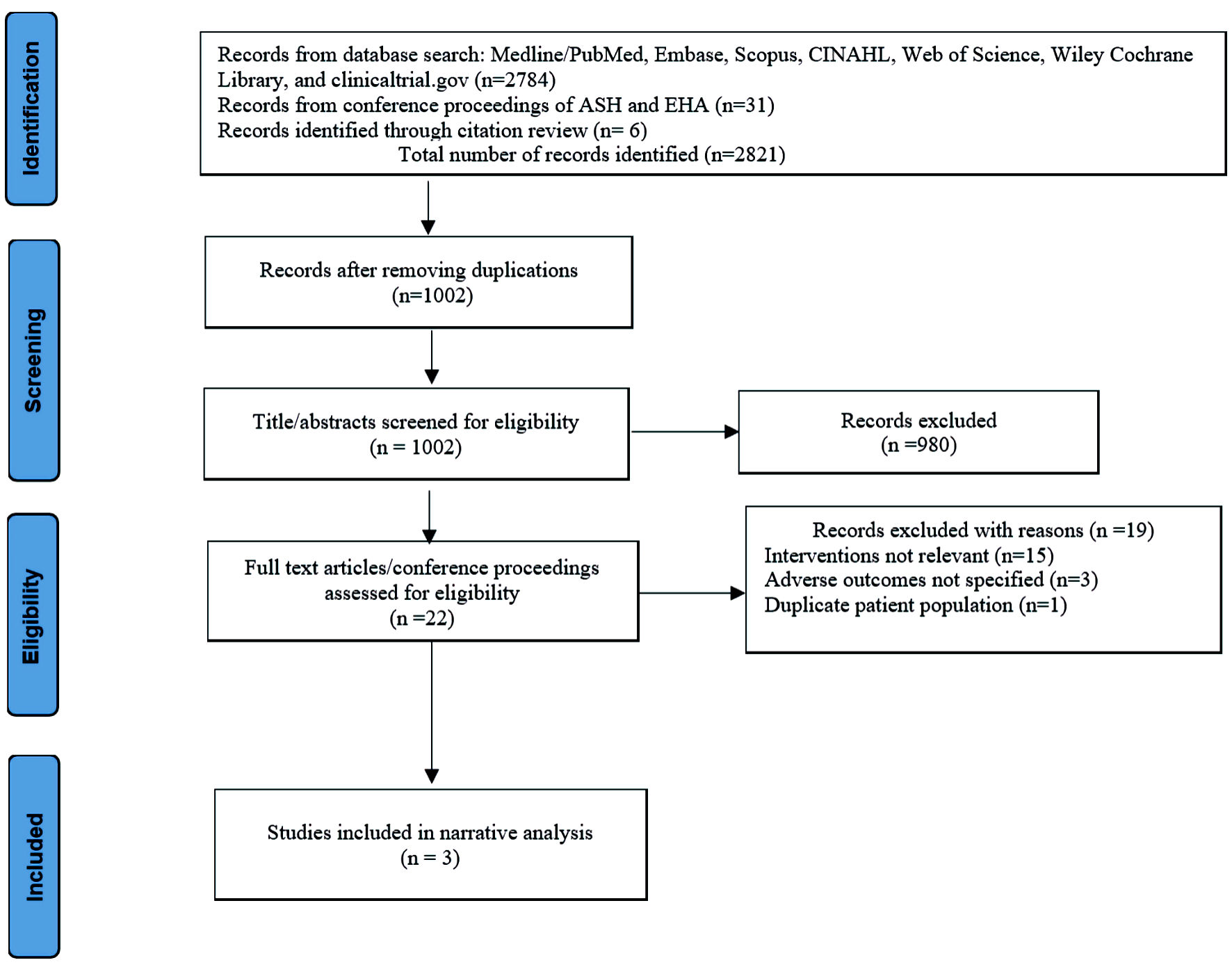 Click for large image | Figure 1. Summary of records identification, screening, eligibility, and inclusion per PRISMA guideline. PRISMA: the preferred reporting items for systematic reviews and meta-analyses. EHA: European Hematology Association; ASH: American Society of Hematology. |
 Click to view | Table 1. Characteristics of the Included Studies |
Study demographics
A total of three studies were included in this review involving 549 SCD patients who were hospitalized for VOC [19-21]. The number of VOC encounters was higher than the number of patients due to the inclusion of recurrent episodes of hospitalization for VOC by the same patient. HbSS is the most prevalent hemoglobin genotype across the studies. Both pediatric and adult SCD patients were included in the studies.
Pain control
Only one study explored the relationship between normal saline bolus administration and sickle cell crisis pain control [19]. Out of 400 SCD patients who were managed for VOC, 261 received normal saline bolus in the ED while 137 did not receive normal saline bolus. Mean baseline pain at presentation (on a pain scale of 0 to 10) was similar between both groups, 8.0 (standard deviation (SD) = 2.1) vs. 8.0 (SD = 2.1) (P = 0.71). At ED disposition, the improvement in pain score was less in the normal saline bolus group when compared to those who did not receive normal saline bolus (2.2 vs. 3.0; P = 0.03). Similarly, admission to inpatient care was higher in the normal saline bolus group (71% vs. 59%; P = 0.01).
Volume overload
The incidence of volume overload following IVF therapy for VOC was explored by one study involving 100 SCD patients in 230 VOC encounters [21]. Each patient received 3 L/24 h (adults) and 3 L/m2/24 h (pediatrics) which was tapered or stopped after 72 h. The primary outcome measure, volume overload was defined by the presence of at least one of lung edema on chest X-ray, peripheral edema or weight gain accompanied by one or more of lung crackles, shortness of breath and/or oxygen requirement, reduction or discontinuation of IVF and/ or use of diuretics. Out of 100 patients, 21 (21%) developed volume overload. The average duration of hospitalization was longer among those who developed volume overload (6 days vs. 4 days; P = 0.037). The independent predictors of volume overload were lactate dehydrogenase level on admission (P = 0.011), history of volume overload (P = 0.017), and blood transfusion during admission (P = 0.005).
New oxygen requirement, acute chest syndrome (ACS), and acute kidney injury
One study provided information on the risk of new oxygen requirement, ACS, and acute kidney injury [20]. Overall, 49 patients made up of 157 VOC encounters were evaluated. The mean volume of IVF (including blood) given was 7.7 L (SD = 9.7) over a median hospital stay of 4 days (interquartile range (IQR): 2 - 7). New oxygen requirement was reported in 20 (20%) of patients who received more than 3 L of IVF compared to eight (14%) among those who received less than 3 L (P = 0.311). Similarly, ACS was more common among those who received more than 3 L, 10 (10%) compared to those who received less than 3 L, two (3.5%) (P = 0.212). In addition, acute kidney injury was reported in two (2.0%) of patients who received more than 3 L and one (1.7%) of those who received less than 3 L (P = 0.99). Although these were not statistically significant, the overall risk of any adverse events was higher in those who received more than 3 L (P = 0.029).
| Discussion | ▴Top |
For a disease affecting millions of people globally with millions of ED presentation and hospitalizations annually, it is surprising there are scanty literature and no randomized controlled trial that evaluated the efficacy and safety of the current practice of IVF use in VOC management. One non-randomized study by Hatch et al in 1965 suggests vigorous IVF hydration is required in VOC management due to insufficiency of oral hydration, while another study by Guy et al in 1971 suggested that an infusion of a large volume of hypotonic fluid to induce hyponatremia improves vaso-occlusion [7, 22]. More recently, a study compared the admission rate and pain control following the infusion of saline warmed to 37.5 °C in the ED compared to those who received non-warmed saline (22 - 24 °C) and reported a similar admission rate and pain control between both groups [23]. The methodological limitations of these studies make them unsuitable for generalized application. As a result, we did not find any clinical trial in the literature that adequately assessed the role of IVF use in the management of VOC.
The practice of fluid administration varies across the included studies with normal saline being the most commonly used fluid, either as boluses or continuous infusions, regardless of the volume status of the patients. However, the use of normal saline may be problematic for SCD patients [19, 21]. Normal saline has a slightly higher osmolarity compared to plasma (308 vs. 290 mOsm/L) with an acidic pH of 5.6 (4.5 to 7.0) [24]. Hyposthenuria and varying degrees of kidney dysfunction (sickle cell nephropathy) are common among SCD patients and may be unable to excrete such a huge solute load, increasing their risk of volume overload [25]. In addition, the use of hyperosmolar fluid has an implication for RBC rheology in VOC. For a disease process provoked primarily by cellular dehydration and increased cellular HbS concentration, an increase in plasma osmolarity has been shown to worsen sickle RBC dehydration, leading to HbS polymerization, reduced cellular deformability, increased adhesion to vascular endothelium, and vaso-occlusion [26]. Also, normal saline infusion may result in iatrogenic hyperchloremic acidosis, which can precipitate hemoglobin polymerization [27, 28]. This may be responsible for the worse pain control among individuals who received normal saline bolus reported in one of the studies [19]. This argues against the use of normal saline in the management of VOC and has led to increasing advocacy for the use of hypotonic fluids rather than normal saline [14]. Negative outcomes following normal saline bolus have also been reported in clinical trials among non-SCD patients. The Fluid Expansion as Supportive Therapy (FEAST) trial showed increased 48-h mortality in acutely ill children who received normal saline bolus [29]. Other trials have reported increased adverse kidney events following the use of normal saline boluses in the ED in critically ill and non-critically ill patients [30, 31].
Regardless of the IVF composition, the use of IVF is a risk factor for volume overload, a commonly encountered problem in the hospital setting. Manifesting as cutaneous and pulmonary edema, volume overload is a major cause of morbidity and mortality in hospitalized patients [32]. The reported incidence of volume overload among patients hospitalized for VOC in the study by Gaartman et al is 21% [21]. In that study, the volume of IVF patients received was 3 L/24 h for adults and 3 L/m2/24 h for children. A report by the UK National Clinical Guideline Center (NCGC) found that one in five (20%) patients who received IVFs suffer complications due to inappropriate administration [33]. In addition, any expansion of the intravascular volume by 2 - 3 L is sufficient to cause volume overload [33]. One of the major underlying factors for volume overload is the failure of providers to administer fluids based on the patient’s volume status and history of volume overload rather than the use of a blanket regimen for all patients with VOC [21]. For SCD patients with varying degrees of renal (hyposthenuria, sickle cell nephropathy), cardiac (diastolic dysfunction), and pulmonary (pulmonary hypertension) dysfunction, volume overload may have severe consequences [25, 34]. In a retrospective review of autopsy studies on 21 SCD patients who died unexpectedly, pulmonary edema was the most common pathologic finding, occurring in 48% of the patients [35]. More than 60% of the patients in that study were initially hospitalized for painful crisis and respiratory failure was the leading cause of death among the patients. Another autopsy study on 36 SCD patients found pulmonary edema in 23 (64%) of the patients [36]. In the absence of well-defined guidelines on IVF use that are based on strong clinical evidence from randomized controlled trials in SCD patients, the practice of IVF use in VOC may continue to be dictated by individual provider experiences with an attending risk of volume overload.
ACS is the leading cause of mortality in SCD and the second leading cause of hospitalizations [37]. ACS is characterized by fever and/or respiratory symptoms such as cough, wheezing, chest pain, and new oxygen requirement accompanied by new infiltrates on chest X-ray [38]. About half of patients with ACS presented initially to the hospital with VOC and later developed this complication during hospitalization with the onset of symptoms occurring about 24 to 72 h after the onset of painful crisis [37]. Although the most recognized underlying etiologies for ACS are lung infection, bone marrow fat embolism, alveolar hypoventilation, and pulmonary infarction secondary to sequestration of sickle RBCs in the pulmonary vasculature, there is evidence to suggest a possible contribution from IVF use [20, 37]. None of the studies included in this review demonstrated a strong association between the use of IVF and the risk of developing ACS, although the study by Gaut et al is suggestive of a potential association [20]. Hypothetically, the use of a hypertonic and acidic fluid such as normal saline could potentially worsen red sickling, thereby contributing to the intravascular pulmonary sequestration of sickle cells. In addition, pulmonary edema, a potential complication of any IVF administration could cause alveolar hypoventilation, hypoxia (with symptoms similar to ACS), and a vicious cycle of sickling of RBCs [39]. However, since ACS often develops after hospitalization for VOC, it is hard to determine the role of in-hospital interventions in provoking this condition. Additional studies are needed to fully clarify the association.
Acute kidney injury is a common finding among SCD patients during VOC episodes and the etiology is thought to be multifactorial [40]. Baddam et al evaluated 197 admissions for VOC and found AKI in 33 patients (17%) [41]. In that study, half of the AKI developed during hospitalization. While the use of non-steroidal anti-inflammatory drugs (NSAID), sepsis, and hypovolemia are established risk factors for AKI in hospitalized patients, it is logical to think that the use of IVFs in the treatment of VOC should reduce the risk of AKI in SCD patient’s hospitalized for VOC [42]. However, there is evidence to suggest inappropriate use of IVF may contribute to the risk of AKI in SCD patients [20]. Hypervolemia and volume overload cause intrarenal venous congestion and have been shown to induce a greater degree of renal injury than that from reduced arterial flow [43]. This is further highlighted by the findings of the Isotonic Solutions and Major Adverse Renal Events Trial (SMART) and the Saline Against Lactated Ringer’s or Plasma-Lyte in the ED (SALT-ED) trial, both of which demonstrated increased adverse kidney events including AKI following the use of normal saline when compared to other balanced crystalloids in critically ill and non-critically ill patients [30, 31]. This underscores the need for judicious use of IVF in SCD patients who are at a higher risk of renal dysfunction compared to the general population.
Although there are emerging advances in the prevention and management of VOC in SCD patients, there are many unanswered questions about the use of IVFs in the management of VOC. Do patients need IVF supplementation or is oral rehydration sufficient? What are the metrics for determining who needs oral vs. IVFs? What is the ideal choice of fluid management: isotonic vs. hypotonic fluids? If hypotonic, what is the ideal option of hypotonic fluid; 0.65% normal saline vs. 0.45% normal saline vs. 0.22% normal saline vs. free water? Randomized controlled trials are needed to address these questions.
There are several limitations of this study that must be taken into consideration. All the included studies are retrospective cohort studies. As such, they are subject to the inherent bias associated with a retrospective cohort study design such as selection bias, detection bias, and reporting bias. Also, it is not possible to establish direct causal effects between IVF administration and the studied adverse outcomes due to the presence of other potential confounders which were not accounted for in the included studies. In addition, there is significant heterogeneity in the measurement of outcomes precluding a meta-analysis.
| Conclusions | ▴Top |
There is a wide variation in the practice of IVF administration in the treatment of VOC in SCD patients. Normal saline, a frequently used IVF for VOC may be associated with adverse outcomes such as worse pain control and volume overload. While hypotonic fluids are theoretically better choices of IVF for VOC, there are no randomized controlled trials on their efficacy and safety in this patient population. IVF use should be tailored to individual characteristics of each SCD patient. Volume overload, new oxygen requirement, ACS and acute kidney injury are major concerns with inappropriate IVF use in SCD patients. Well-defined evidence-based guidelines on IVF emanating from randomized controlled trials are required to fully clarify the place of IVF in the management of VOC.
| Supplementary Material | ▴Top |
Suppl 1. Search strategy.
Suppl 2. Assessment of study quality based on the Newcastle-Ottawa scale.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
AO conceived and designed the study. AO and WA collected and interpreted all relevant data. AO, SO, WA, OO and DL prepared the manuscript. All authors read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020;92(9):1549-1555.
doi pubmed - Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311-323.
doi - Aygun B, Odame I. A global perspective on sickle cell disease. Pediatr Blood Cancer. 2012;59(2):386-390.
doi pubmed - Abd El-Ghany SM, Tabbakh AT, Nur KI, Abdelrahman RY, Etarji SM, Almuzaini BY. Analysis of causes of hospitalization among children with sickle cell disease in a group of private hospitals in Jeddah, Saudi Arabia. J Blood Med. 2021;12:733-740.
doi pubmed - Fingar KR, Owens PL, Reid LD, Mistry KB, Barrett ML. Characteristics of inpatient hospital stays involving sickle cell disease, 2000-2016. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD), 2006.
- Lanzkron S, Carroll CP, Haywood C, Jr. The burden of emergency department use for sickle-cell disease: an analysis of the national emergency department sample database. Am J Hematol. 2010;85(10):797-799.
doi pubmed - Hatch FE, Diggs LW. Fluid Balance in Sickle-Cell Disease. Arch Intern Med. 1965;116:10-17.
doi pubmed - Brugnara C. Sickle cell dehydration: Pathophysiology and therapeutic applications. Clin Hemorheol Microcirc. 2018;68(2-3):187-204.
doi pubmed - Nagalla S, Ballas SK. Drugs for preventing red blood cell dehydration in people with sickle cell disease. Cochrane Database Syst Rev. 2010;1:CD003426.
doi - Sunshine HR, Hofrichter J, Eaton WA. Requirement for therapeutic inhibition of sickle haemoglobin gelation. Nature. 1978;275(5677):238-240.
doi pubmed - Mozzarelli A, Hofrichter J, Eaton WA. Delay time of hemoglobin S polymerization prevents most cells from sickling in vivo. Science. 1987;237(4814):500-506.
doi pubmed - Ferrone FA, Hofrichter J, Eaton WA. Kinetics of sickle hemoglobin polymerization. II. A double nucleation mechanism. J Mol Biol. 1985;183(4):611-631.
doi - Rosa RM, Bierer BE, Thomas R, Stoff JS, Kruskall M, Robinson S, Bunn HF, et al. A study of induced hyponatremia in the prevention and treatment of sickle-cell crisis. N Engl J Med. 1980;303(20):1138-1143.
doi pubmed - Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, Jordan L, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033-1048.
doi pubmed - Carden MA, Fay ME, Lu X, Mannino RG, Sakurai Y, Ciciliano JC, Hansen CE, et al. Extracellular fluid tonicity impacts sickle red blood cell deformability and adhesion. Blood. 2017;130(24):2654-2663.
doi pubmed - Carden MA, Patil P, Ahmad ME, Lam WA, Joiner CH, Morris CR. Variations in pediatric emergency medicine physician practices for intravenous fluid management in children with sickle cell disease and vaso-occlusive pain: A single institution experience. Pediatr Blood Cancer. 2018;65(1):e26742.
doi pubmed - Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
doi pubmed - Ding S, Qiao X, Kucera GL, Bierbach U. Using a build-and-click approach for producing structural and functional diversity in DNA-targeted hybrid anticancer agents. J Med Chem. 2012;55(22):10198-10203.
doi pubmed - Carden MA, Brousseau DC, Ahmad FA, Bennett J, Bhatt S, Bogie A, Brown K, et al. Normal saline bolus use in pediatric emergency departments is associated with poorer pain control in children with sickle cell anemia and vaso-occlusive pain. Am J Hematol. 2019;94(6):689-696.
doi pubmed - Gaut D, Jones J, Chen C, Ghafouri S, Leng M, Quinn R. Outcomes related to intravenous fluid administration in sickle cell patients during vaso-occlusive crisis. Ann Hematol. 2020;99(6):1217-1223.
doi pubmed - Gaartman AE, Sayedi AK, Gerritsma JJ, de Back TR, van Tuijn CF, Tang MW, Heijboer H, et al. Fluid overload due to intravenous fluid therapy for vaso-occlusive crisis in sickle cell disease: incidence and risk factors. Br J Haematol. 2021;194(5):899-907.
doi pubmed - Guy RB, Gavrilis PK, Rothenberg SP. In vitro and in vivo effect of hypotonic saline on the sickling phenomenon. Am J Med Sci. 1973;266(4):267-277.
doi pubmed - Quarrie RP, Stoner MJ, Choueiki JM, Bonsu BK, Cohen DM. Clinical impact of warmed intravenous saline in sickle cell patients with vasoocclusive episodes. Pediatr Emerg Care. 2020;36(5):229-235.
doi pubmed - Ding T, Zhang J, Wang T, Cui P, Chen Z, Jiang J, Zhou S, et al. Potential influence of menstrual status and sex hormones on female severe acute respiratory syndrome coronavirus 2 infection: a cross-sectional multicenter study in Wuhan, China. Clin Infect Dis. 2021;72(9):e240-e248.
doi pubmed - Lemes RPG, Rocha Laurentino M, Castelo LR, Silva Junior G. Sickle cell disease and the kidney: pathophysiology and novel biomarkers. Contrib Nephrol. 2021;199:114-121.
doi pubmed - Carden MA, Fay M, Sakurai Y, McFarland B, Blanche S, DiPrete C, Joiner CH, et al. Normal saline is associated with increased sickle red cell stiffness and prolonged transit times in a microfluidic model of the capillary system. Microcirculation. 2017;24(5):e12353.
doi pubmed - Bookchin RM, Balazs T, Landau LC. Determinants of red cell sickling. Effects of varying pH and of increasing intracellular hemoglobin concentration by osmotic shrinkage. J Lab Clin Med. 1976;87(4):597-616.
- Guidet B, Soni N, Della Rocca G, Kozek S, Vallet B, Annane D, James M. A balanced view of balanced solutions. Crit Care. 2010;14(5):325.
doi pubmed - Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483-2495.
doi pubmed - Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, Slovis CM, et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. N Engl J Med. 2018;378(9):819-828.
doi pubmed - Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, Stollings JL, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839.
doi pubmed - Van Regenmortel N, Moers L, Langer T, Roelant E, De Weerdt T, Caironi P, Malbrain M, et al. Fluid-induced harm in the hospital: look beyond volume and start considering sodium. From physiology towards recommendations for daily practice in hospitalized adults. Ann Intensive Care. 2021;11(1):79.
doi pubmed - National Clinical Guideline Centre (UK). Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital [Internet]. London: Royal College of Physicians (UK); 2013 Dec. (NICE Clinical Guidelines, No. 174.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK247761/.
- Gladwin MT, Sachdev V. Cardiovascular abnormalities in sickle cell disease. J Am Coll Cardiol. 2012;59(13):1123-1133.
doi pubmed - Graham JK, Mosunjac M, Hanzlick RL, Mosunjac M. Sickle cell lung disease and sudden death: a retrospective/prospective study of 21 autopsy cases and literature review. Am J Forensic Med Pathol. 2007;28(2):168-172.
doi pubmed - Oppenheimer EH, Esterly JR. Pulmonary changes in sickle cell disease. Am Rev Respir Dis. 1971;103(6):858-859.
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359(21):2254-2265.
doi pubmed - Howard J, Hart N, Roberts-Harewood M, Cummins M, Awogbade M, Davis B, Committee B. Guideline on the management of acute chest syndrome in sickle cell disease. Br J Haematol. 2015;169(4):492-505.
doi pubmed - Vadasz I, Sznajder JI. Gas exchange disturbances regulate alveolar fluid clearance during acute lung injury. Front Immunol. 2017;8:757.
doi pubmed - Mammen C, Bissonnette ML, Matsell DG. Acute kidney injury in children with sickle cell disease-compounding a chronic problem. Pediatr Nephrol. 2017;32(8):1287-1291.
doi pubmed - Baddam S, Aban I, Hilliard L, Howard T, Askenazi D, Lebensburger JD. Acute kidney injury during a pediatric sickle cell vaso-occlusive pain crisis. Pediatr Nephrol. 2017;32(8):1451-1456.
doi pubmed - Finlay S, Bray B, Lewington AJ, Hunter-Rowe CT, Banerjee A, Atkinson JM, Jones MC. Identification of risk factors associated with acute kidney injury in patients admitted to acute medical units. Clin Med (Lond). 2013;13(3):233-238.
doi pubmed - Ding X, Cheng Z, Qian Q. Intravenous fluids and acute kidney injury. Blood Purif. 2017;43(1-3):163-172.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


