| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 12, Number 2, April 2023, pages 66-74
Incidence and Risk of Hematological Adverse Events Associated With Immune Checkpoint Inhibitors: A Systematic Literature Review and Meta-Analysis
Takuma Ohashia, d, Kaoru Takase-Minegishia, Ayaka Maedaa, Naoki Hamadaa, Ryusuke Yoshimia, Yohei Kirinoa, Hiroshi Teranakaa, Hiroyoshi Kunimotoa, Maki Hagiharaa, Kenji Matsumotoa, Ho Namkoongb, Nobuyuki Horitac, Hideaki Nakajimaa
aDepartment of Stem Cell and Immune Regulation, Yokohama City University Graduate School of Medicine, Yokohama, Japan
bDepartment of Infectious Diseases, Keio University School of Medicine, Tokyo, Japan
cChemotherapy Center, Yokohama City University Hospital, Yokohama, Japan
dCorresponding Author: Takuma Ohashi, Department of Stem Cell and Immune Regulation, Yokohama City University Graduate School of Medicine, Yokohama 236-0004, Japan
Manuscript submitted February 6, 2023, accepted March 25, 2023, published online April 30, 2023
Short title: Hematological AEs Associated With ICIs
doi: https://doi.org/10.14740/jh1090
| Abstract | ▴Top |
Background: Immune checkpoint inhibitors (ICIs) have been a breakthrough in cancer therapy. ICI therapy is generally better tolerated than cytotoxic chemotherapy; however, hematological adverse events (AEs) have not been fully analyzed. Hence, we performed a meta-analysis to evaluate the incidence and risk of ICI-related hematological AEs.
Methods: A systematic literature search was performed using PubMed, EMBASE, Cochrane Library, and the Web of Science Core Collection. Phase III randomized controlled trials (RCTs) involving ICI combination regimens were selected. The experimental group received ICIs with systemic treatment, and the control group received only the same systemic treatment. Odds ratios (ORs) for anemia, neutropenia, and thrombocytopenia were calculated using a random-model meta-analysis.
Results: We identified 29 RCTs with 20,033 patients. The estimated incidence rates for anemia of all grades and grades III-V were 36.5% (95% confidence interval (CI) 30.23 - 42.75) and 4.1% (95% CI 3.85 - 4.42), respectively. The incidence of neutropenia (all grades 29.7%, grades III-V 5.3%) and thrombocytopenia (all grades 18.0%, grades III-V 1.6%) was also calculated.
Conclusion: Treatment with ICIs seemed unlikely to increase the incidence of anemia, neutropenia, and thrombocytopenia in all grades. However, programmed cell death-1 receptor ligand inhibitors significantly increased the risk of grades III-V thrombocytopenia (OR 1.53; 95% CI 1.11 - 2.11). Further research is needed to examine the potential risk factors.
Keywords: Immune checkpoint inhibitor; Hematological toxicities; Immune-related adverse events; Meta-analysis
| Introduction | ▴Top |
Immune checkpoint inhibitors (ICIs) have shown significant efficacy in various cancers, including non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), melanoma, classical Hodgkin lymphoma, head and neck squamous cell carcinoma, urothelial cancer, and renal cell carcinoma [1-3]. Approved ICIs include monoclonal antibodies against programmed cell death-1 receptor (PD-1), its ligand (PD-L1), and cytotoxic T lymphocyte antigen-4 (CTLA-4). In addition to the consistent and significant clinical effects achieved by enhancing the immune response to control malignancies, ICIs also induce severe multiple organ system toxicities. These include hematopoietic system, which may cause intolerance resulting in an immune response against autologous tissue [4, 5]. These adverse events (AEs), termed immune-related AEs (irAEs), are primarily associated with dysregulation of T-cell function. Frequently reported clinical hematological complications include neutropenia, immune thrombocytopenia (ITP), autoimmune hemolytic anemia, and immune thrombocytopenic purpura. Generally, hematological side effects associated with conventional chemotherapies can be managed with appropriate supportive care; however, AEs from ICIs can be severe and, if neglected, can lead to conditions that have poor prognosis.
Although multicenter clinical data analyses, case series analyses, and meta-analyses have been conducted on the hematological AEs of ICI therapies [6-8], to the best of our knowledge, no meta-analysis using randomized controlled trials (RCTs) has focused on concomitant anticancer therapies. ICIs are being extensively used for a wide range of cancers, in combination with other anticancer agents or in two-drug regimens. Hematological complications, such as anemia, can occur in patients with advanced cancer; hence, it is important to determine the hematological toxicities associated with ICIs for the selection of treatment regimens and management of AEs. Therefore, we performed a systematic review and meta-analysis of RCTs to estimate the incidence of hematological AEs and their association with ICIs.
| Materials and Methods | ▴Top |
Systematic search for locating studies and selection
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9, 10] and registered in the University Hospital Medical Information Network Center Clinical Trial Registry (Japan) (UMIN000046032) [11]. Institutional review board approval and patient informed consent were waived since this was a review study. This study was conducted in compliance with all the applicable institutional ethical guidelines for the care.
In the electronic search, we systematically queried PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and the Web of Science Core Collection (up to August 16, 2021) for RCTs reporting anemia, neutropenia, and thrombocytopenia with ICIs. The search formulas are presented in Supplementary Material 1 (www.thejh.org). Two investigators (AM and KT-M) independently screened the candidate articles by checking the title and abstract after uploading the citation list into Endnote X9 software (Thomson Reuters, Philadelphia, PA, USA). The inclusion criteria were as follows: 1) patients clinically diagnosed with hematological cancer or other solid tumors; 2) ICI monotherapy with systemic treatment as the experimental group and only the same systemic treatment for the control group; 3) study with three arms where ICI was included in at least one arm; 4) published study designed as a phase III RCT; and 5) evaluation of hematological AEs in the study. The exclusion criteria were as follows: 1) studies with no data on anemia, neutropenia, and thrombocytopenia; 2) republished research literature; 3) studies published in languages other than English; and 4) conference abstracts. Disagreements in assessing the cases or data were resolved via discussion between the two investigators.
Data extraction
The total number of patients treated with ICIs and the number of patients who developed grade I-V anemia, neutropenia, and thrombocytopenia (Supplementary Material 2, www.thejh.org) in each treatment arm were collected from the eligible RCTs. If a study included more than two comparable arms, we selected only one comparable pair to evaluate the effect of ICIs. We also included grade ≥ 3 anemia, neutropenia, and thrombocytopenia. AEs were graded according to the Common Terminology Criteria for Adverse Events system [12]. Data were extracted in standardized tables by one reviewer (TO) and verified by a second reviewer (AM).
Data synthesis and statistical analyses
A meta-analysis evaluating the contribution of ICIs to the incidence of hematological AEs was performed using random-effects models. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for the risk of hematological toxicity associated with ICIs. Heterogeneity was assessed using the I2 statistic. Heterogeneity was indicated by I2, where 0% indicated no heterogeneity and 100% indicated the strongest heterogeneity. Statistical analyses were performed using Review Manager 5.4 (Cochrane Community, London, UK). We also conducted subgroup analyses based on ICI type (PD-1, PD-L1 inhibitor and CTLA-4 inhibitor) and cancer type. In addition, we assessed the risk of hematological toxicity between ICI + cytotoxic agents and ICI + non-cytotoxic agents. The Cochrane Risk of Bias Tool was used to evaluate the risk of bias for each RCT [13]. Two reviewers (TO and AM) independently assessed RCT quality.
| Results | ▴Top |
Summary of systematic review and meta-analysis
Of the 5,471 candidate articles, 120 were reviewed in detail (Fig. 1). Of the 120 articles, 19 were excluded, and 71 of the 101 selected articles had insufficient data to assess ICI-related hematological AEs. Finally, 29 RCTs were included in this meta-analysis (Supplementary Material 3, www.thejh.org) [14-42]. The characteristics of the included studies are summarized in Supplementary Material 3 (www.thejh.org). The overall risk of bias for most evaluated studies was low (Supplementary Material 4, www.thejh.org). Visual inspection of funnel plots was also assessed (Supplementary Material 5, www.thejh.org). There was no clear risk of publication bias in the included studies.
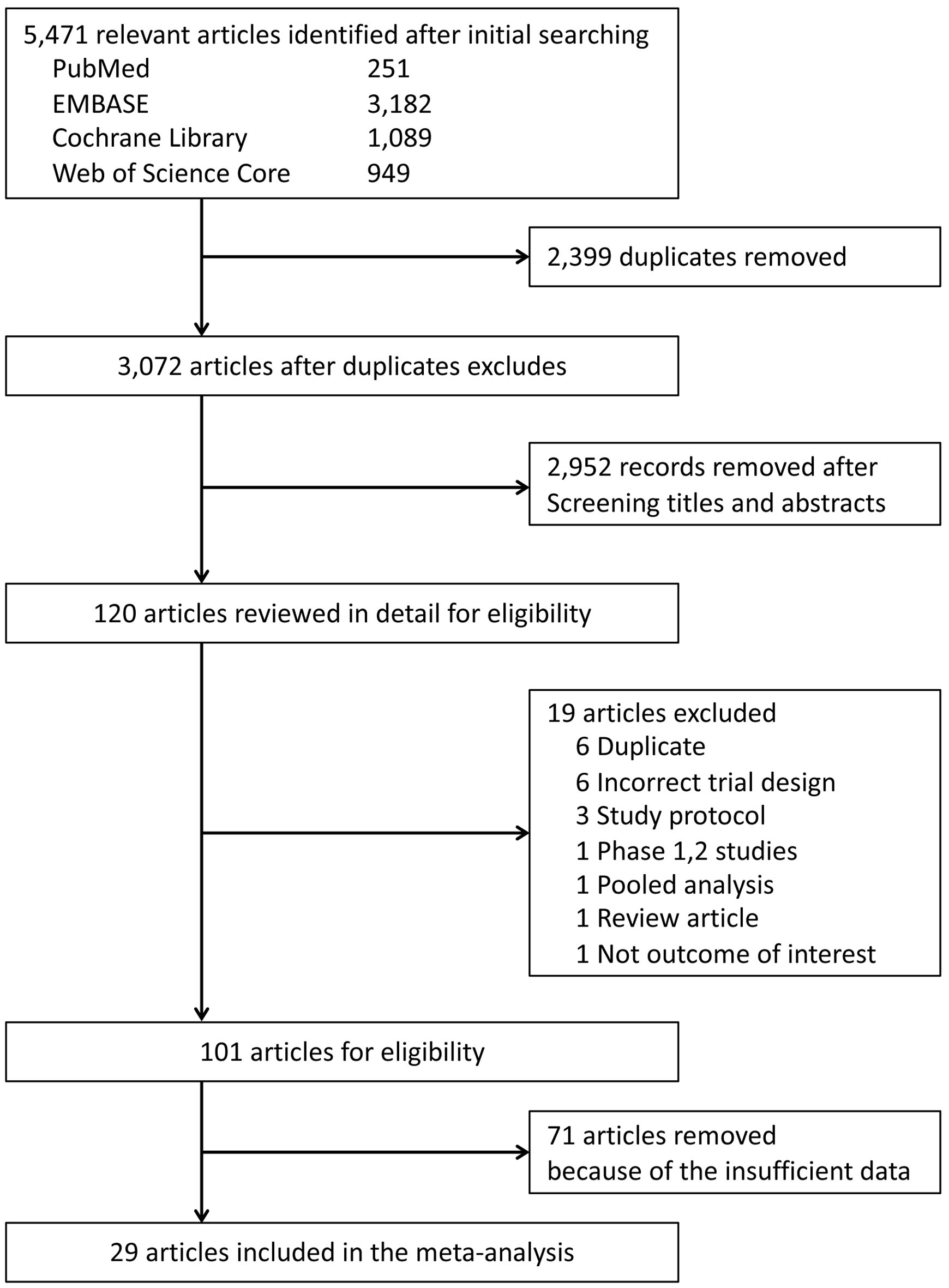 Click for large image | Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study selection process. |
The PD-1 inhibitor was used in 10 studies involving 3,901 patients, the PD-L1 inhibitor was evaluated in 14 studies involving 4,713 patients, and the CTLA-4 inhibitor was assessed in five studies involving 2,057 patients. Tumor types of the eligible studies included NSCLC (n = 7), breast cancer (n = 5), gastric cancer (n = 4), SCLC (n = 3), myeloma (n = 3), urothelial cancer (n = 3), melanoma (n = 2), and ovarian cancer (n = 2).
Incidence of hematological complications treated with ICIs
The incidence of anemia, neutropenia, and thrombocytopenia for ICIs with subsequent systemic therapy was analyzed. A total of 29 RCTs involving 20,033 patients with grade I-V anemia and 28 RCTs involving 19,522 patients with grade III-V anemia were evaluated. The estimated incidence rates for grades I-V and III-V anemia were 36.5% (95% CI 30.2 - 42.8) and 4.1% (95% CI 3.9 - 4.4), respectively (Fig. 2).
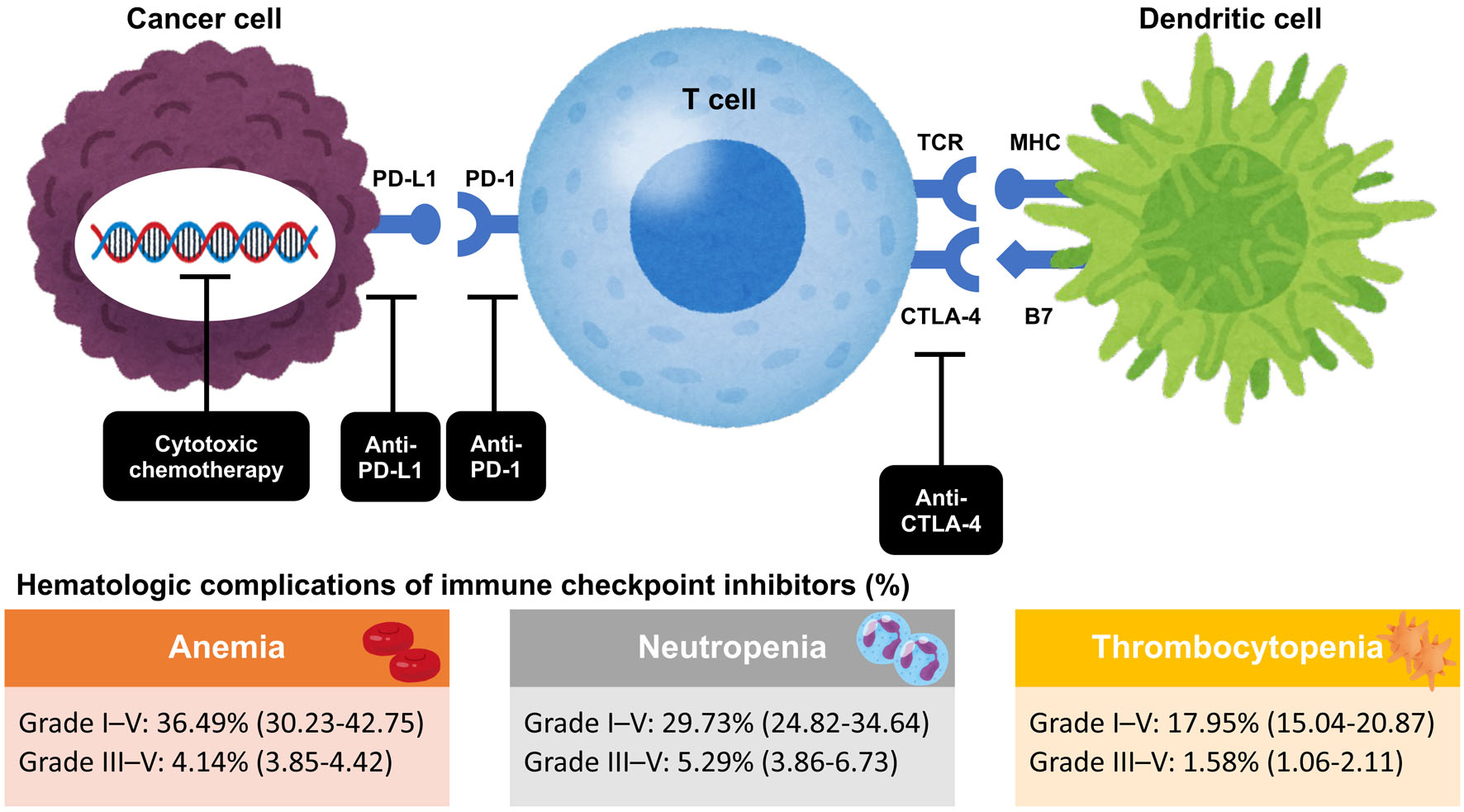 Click for large image | Figure 2. Mechanism of action of immune checkpoint inhibitors and hematological complications of immune checkpoint inhibitors. CTLA-4: cytotoxic T lymphocyte antigen-4; MHC: major histocompatibility complex; PD-1: programmed cell death-1 receptor; PD-L1: PD-1 ligand 1; TCR: T-cell receptor. |
Regarding neutropenia, 25 RCTs involving 17,536 patients and 26 RCTs involving 18,310 patients were evaluated for grade I-V and III-V neutropenia. The estimated incidence rates for grade I-V and grade III-V neutropenia were 29.7% (95% CI 24.8 - 34.6) and 5.3% (95% CI 3.9 - 6.7), respectively (Fig. 2). In subgroup analyses based on ICI type, no significant difference in heterogeneity was observed for grade I-V neutropenia (P = 0.69).
Regarding thrombocytopenia, 18 RCTs involving 12,575 patients and 23 RCTs involving 15,562 patients were evaluated for grade I-V and III-V thrombocytopenia. The estimated incidence rates for grade I-V and grade III-V thrombocytopenia were 18.0% (95% CI 15.0 - 20.1) and 1.6% (95% CI 1.1 - 2.1), respectively (Fig. 2).
Risk of hematological complications associated with ICI treatment
The risk of anemia, neutropenia, and thrombocytopenia in each RCT that compared ICI plus systemic treatment with the same systemic treatment only as controls was evaluated. Compared with the control arms, treatment with ICIs did not increase the incidence of grade I-V anemia (OR 1.05; 95% CI 0.98 - 1.14, I2 = 26%) (Fig. 3). The ORs of grade I-V neutropenia and thrombocytopenia were 1.10 (95% CI 1.01 - 1.19, I2 = 23%) and 1.15 (95% CI 1.02 - 1.31, I2 = 37%), respectively (Fig. 3). We also analyzed the data based on ICI types. For grade I-V anemia, neutropenia, and thrombocytopenia, no differences were observed among the ICI types (P > 0.05) (Supplementary Material 6, www.thejh.org).
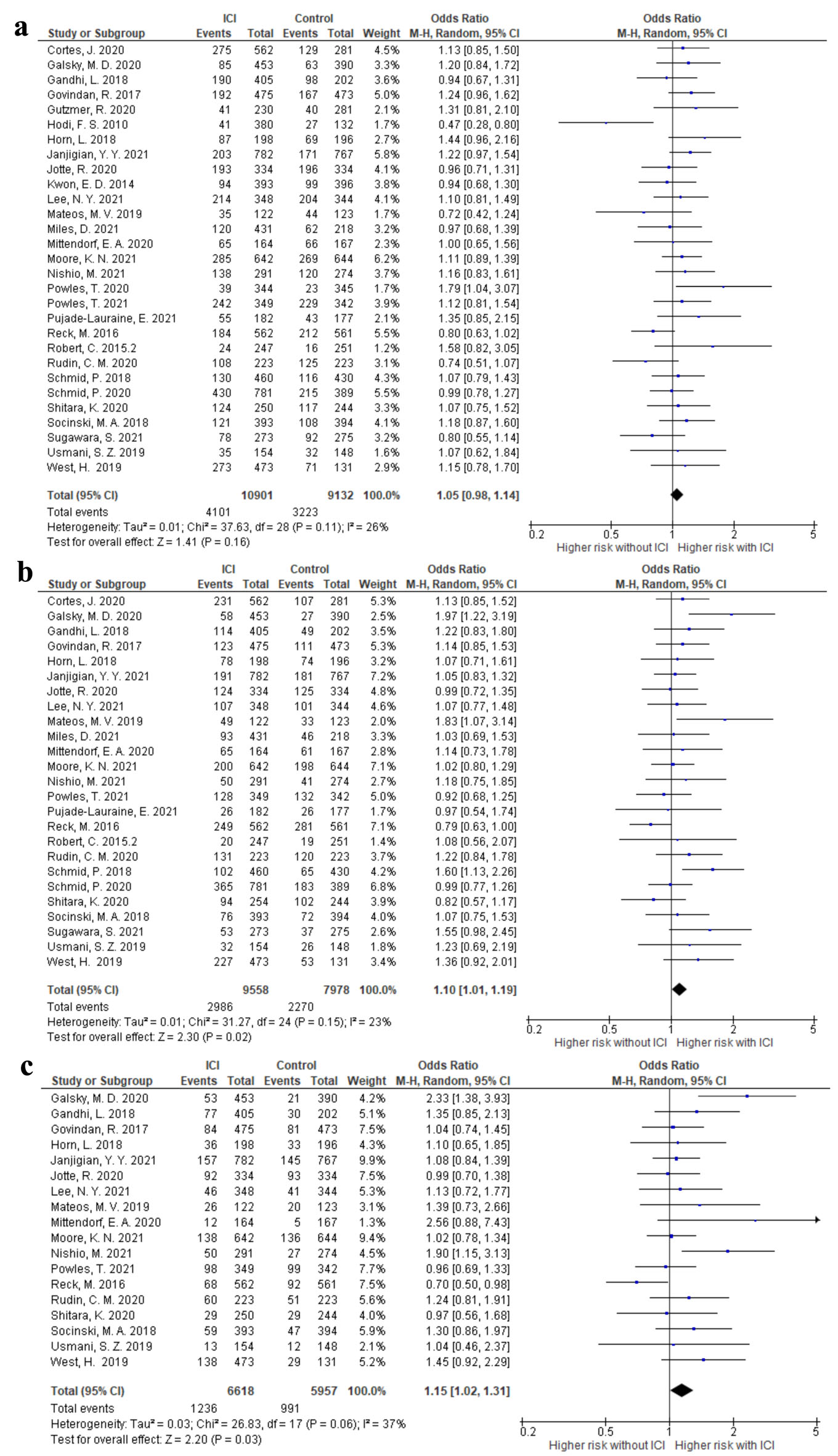 Click for large image | Figure 3. The forest plot of the odds ratio of grade I-V hematological adverse events comparing the additional immune checkpoint inhibitor use with control therapy. (a) Anemia. (b) Neutropenia. (c) Thrombocytopenia. CI: confidence interval. |
Cases of severe or life-threatening anemia, neutropenia, and thrombocytopenia were infrequent, but their frequency varied depending on the type of ICIs and concomitant medication. In grade III-V hematological AEs, CTLA-4 inhibitor and PD-1 inhibitor did not increase the risk; however, PD-L1 inhibitor significantly increased the risk of thrombocytopenia (OR 1.53; 95% CI 1.11 - 2.11, I2 = 0%) (Supplementary Material 7, www.thejh.org). Nevertheless, no significant difference was observed in each group in this analysis (I2 = 38.2%, P = 0.20). Grades III-V thrombocytopenia induced by PD-L1 inhibitor was more frequent when ICIs were combined with cytotoxic agents than in combination with non-cytotoxic agents (Supplementary Material 7, www.thejh.org).
Subgroup analysis of the risk of hematological toxicities based on cancer type
The incidence of hematological AEs did not significantly differ according to the type of cancer. Subgroup analysis revealed that some combinations of ICIs and cancer type increased the incidence of hematological AEs, with a significant increase in grade III to V thrombocytopenia, especially in the combination of PD-L1 and NSCLC (OR 2.85; 95% CI 1.17 - 6.96, I2 = 0%) (Supplementary Material 8, www.thejh.org). However, the small number of RCTs included in individual categories should be interpreted with caution.
| Discussion | ▴Top |
ICIs have revolutionized cancer therapy by providing antitumor effects in various types and stages of cancer that are not achievable with existing drugs. Consequently, many clinical trials are underway to expand their indications. ICI therapies are generally better tolerated, and their associated toxicity can be managed with appropriate supportive care compared to cytotoxic chemotherapy. Agents that inhibit coinhibitory immune checkpoint molecules, such as PD-1, activate the immune response to control malignancy. However, ICIs may also induce an immune response against self-tissues, leading to various AEs. Clinically, this manifests as autoimmune disease-like side effects including skin, gastrointestinal, liver, lung, and endocrine toxicity. These AEs, called irAEs, are primarily associated with dysregulated T-cell functions. Our study analyzed only anemia, neutropenia, and thrombocytopenia due to ICIs. However, other life-threatening hematological irAEs such as aplastic anemia, hemophagocytic lymphohistiocytosis and acquired hemophilia A after ICIs have also been reported [43, 44], but these AEs were not analyzed. We performed a systematic review and meta-analysis of ICI trials in patients with cancer and evaluated the incidence of hematotoxicity in different ICIs, combination of each ICI and other systemic chemotherapy, and tumor types. Hematological abnormalities are frequently seen in cancer patients. The strength of this study was only phase III RCTs that compared the add-on effect of ICIs on the control arm were extracted, resulting in more accurate risk for ICI-related cytopenias.
The mechanisms underlying the risk of immunotherapy-related cytopenia are currently unclear, but multiple mechanisms may be involved. Patients with urothelial cancer or NSCLC may have previously received platinum-based chemotherapy, which may explain the high incidence of cytopenia. Kasamatsu et al reported that patients with chronic ITP had a significantly higher frequency of the PDCD1 +7209 TT genotype, a single nucleotide polymorphism in PD -1, which is possibly related to our results [44]. Drug-related autoimmune phenomena of ICIs are associated with the direct activation of autoreactive T and B cells and suppression of T-reg cells [45]. In the case of anemia, a study reported that the activation of B cell clones with hemolytic action on erythrocytes causes anemia [45]. In the case of platelets, immune-related thrombocytopenia (irTCP), including ITP, caused by ICI, is considered. IrTCP is mediated by platelet autoantibodies, which accelerate platelet destruction and inhibit platelet production. The proclivity to develop platelet-reactive antibodies arises from diverse mechanisms. In this study, it is remarkable that the risk of grade III-V thrombocytopenia was significantly higher than that of other hematological toxicities. Furthermore, we found a significant increase in grade III-V thrombocytopenia in the combination of PD-L1 and NSCLC. Strikingly, this finding is consistent with a previous study [46], which showed platelets, during their frequent interaction with tumor cells, ingest PD-L1 and present it on their surface using platelets from NSCLC patients. Tyler et al also showed that grade III or higher thrombocytopenia caused by ICI also affected overall survival reduction compared with thrombocytopenia caused by other causes, and the median (interquartile) time to grade III thrombocytopenia was 72.5 days [47]. Since several reports have linked the development of irAEs to prolonged OS, irTCP is likely to have a significant impact on the clinical course, and it is important to identify and appropriately manage patients at higher risk [48-53]. Previous studies have reported that smoking status, cancer type (melanoma and lung cancer), body mass index (> 25), and low-grade thrombocytopenia prior to ICI therapy were significantly associated with the development of grade III or higher thrombocytopenia of any etiology and early intervention is required for such patients [48]. Future studies are needed to determine the relationship between response rates for ICIs and cytopenia.
Our study had several limitations. First, it was a study-level meta-analysis and was not based on individual patient data. Consequently, a comprehensive analysis, adjusting for baseline factors such as performance status, age, prior treatment, and other disparities that exist between trials was not possible. Second, the clinical studies included in our analysis included cancers with different levels of risk of hematotoxicity. There were not a sufficient number of RCTs to analyze for each ICI drug. Unfortunately, the newer PD-1 inhibitors (dostarlimab; zimberelimab; sintilimab) were not included in the present analyses because they were not well reported at the time of our literature search. Lastly, hematotoxicity may occur due to a variety of common exposures, medications, and concomitant conditions that influence the platelet count. Past and concurrent chemotherapy are the major causes of mild to life-threatening hematotoxicity.
In conclusion, the present meta-analysis is one of the few attempts to systematically estimate the incidence of hematotoxicity associated with immunotherapy in patients with cancer using a comprehensive search analysis. Treatment with ICIs seemed unlikely to increase the incidence of anemia, neutropenia, and thrombocytopenia in all grades, but PD-L1 inhibitors may be associated with a high risk of grade III-V thrombocytopenia. For the safe use of these drugs, periodic checks and appropriate management of toxicity are required. Further studies are needed to clarify the pathogenesis and risk factors of ICI-associated hematotoxicity.
| Supplementary Material | ▴Top |
Suppl 1. Search formulas.
Suppl 2. Common Terminology Criteria for Adverse Events v5.0 for hematological adverse events.
Suppl 3. Overview of the included studies in the meta-analysis.
Suppl 4. Risk of bias across studies assessed using the Cochrane risk of bias tool.
Suppl 5. Funnel plots assessing publication bias for odds ratio of immune checkpoint inhibitor-induced hematological adverse events.
Suppl 6. Subgroup analysis based on immune checkpoint inhibitor type.
Suppl 7. Result of meta-analysis on odds ratios of grade III-V anemia, neutropenia and thrombocytopenia comparing the additional ICI use with control therapy as per different types of ICIs
Suppl 8. Subgroup analysis based on cancer type.
Acknowledgments
None to declare.
Financial Disclosure
This study did not receive any funding.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
All authors made a substantial contribution to the design of the study, interpreted the data, and reviewed the manuscript; TO, AM and KM performed data extraction and analyses and wrote the first draft. All authors critically revised the paper for important intellectual content, approved the final version, and agreed with the submission.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
ICIs: immune checkpoint inhibitors; AEs: adverse events; RCTs: randomized controlled trials; ORs: odds ratios; CI: confidence interval; NSCLC: non-small cell lung cancer; SCLC: small cell lung cancer; PD-1: programmed cell death-1 receptor; PD-L1: programmed cell death-1 receptor ligand; CTLA-4: cytotoxic T lymphocyte antigen-4; irAE: immune-related AE; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; irTCP: immune-related thrombocytopenia; ITP: immune thrombocytopenia
| References | ▴Top |
- Onoi K, Chihara Y, Uchino J, Shimamoto T, Morimoto Y, Iwasaku M, Kaneko Y, et al. Immune checkpoint inhibitors for lung cancer treatment: a review. J Clin Med. 2020;9(5):1362.
doi pubmed pmc - Lopez-Beltran A, Cimadamore A, Blanca A, Massari F, Vau N, Scarpelli M, Cheng L, et al. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers (Basel). 2021;13(1):131.
doi pubmed pmc - Togasaki K, Sukawa Y, Kanai T, Takaishi H. Clinical efficacy of immune checkpoint inhibitors in the treatment of unresectable advanced or recurrent gastric cancer: an evidence-based review of therapies. Onco Targets Ther. 2018;11:8239-8250.
doi pubmed pmc - Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148.
doi pubmed - Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168.
doi pubmed - Petrelli F, Ardito R, Borgonovo K, Lonati V, Cabiddu M, Ghilardi M, Barni S. Haematological toxicities with immunotherapy in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2018;103:7-16.
doi pubmed - Kramer R, Zaremba A, Moreira A, Ugurel S, Johnson DB, Hassel JC, Salzmann M, et al. Hematological immune related adverse events after treatment with immune checkpoint inhibitors. Eur J Cancer. 2021;147:170-181.
doi pubmed - Wilson NR, Lockhart JR, Garcia-Perdomo HA, Oo TH, Rojas-Hernandez CM. Management and outcomes of hematological immune-related adverse events: systematic review and meta-analysis. J Immunother. 2022;45(1):13-24.
doi pubmed - Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
doi pubmed pmc - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed pmc - UMIN Center. UMIN Clinical Trials Registry (UMIN-CTR). Available at: https://www.umin.ac.jp/ctr/ctrregist.htm.
- Diane MF Savarese. Common terminology criteria for adverse events. Up To Date.
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
doi pubmed pmc - Yang JC, Shepherd FA, Kim DW, Lee GW, Lee JS, Chang GC, Lee SS, et al. Osimertinib plus durvalumab versus osimertinib monotherapy in EGFR T790M-positive NSCLC following previous EGFR TKI therapy: CAURAL brief report. J Thorac Oncol. 2019;14(5):933-939.
doi pubmed - Galsky MD, Arija JAA, Bamias A, Davis ID, De Santis M, Kikuchi E, Garcia-Del-Muro X, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547-1557.
doi pubmed - Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092.
doi pubmed - Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, Han B, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449-3457.
doi pubmed - Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, Pereira RP, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395(10240):1835-1844.
doi pubmed - Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
doi pubmed pmc - Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379(23):2220-2229.
doi pubmed - Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27-40.
doi pubmed pmc - Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, Soo R, et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J Thorac Oncol. 2020;15(8):1351-1360.
doi pubmed - Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700-712.
doi pubmed pmc - Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, Harrington K, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22(4):450-462.
doi pubmed - Mateos MV, Blacklock H, Schjesvold F, Oriol A, Simpson D, George A, Goldschmidt H, et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6(9):e459-e469.
doi pubmed - Miles D, Gligorov J, Andre F, Cameron D, Schneeweiss A, Barrios C, Xu B, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32(8):994-1004.
doi pubmed - Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, Koehler A, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090-1100.
doi pubmed - Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, Myers T, et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol. 2021;39(17):1842-1855.
doi pubmed pmc - Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, Longeras PD, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16(4):653-664.
doi pubmed - Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-1230.
doi pubmed - Powles T, Csoszi T, Ozguroglu M, Matsubara N, Geczi L, Cheng SY, Fradet Y, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931-945.
doi pubmed - Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, Richardson GE, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021;22(7):1034-1046.
doi pubmed - Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, Pietanza MC, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740-3748.
doi pubmed - Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330.
doi pubmed - Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, Cheema PK, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369-2379.
doi pubmed pmc - Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108-2121.
doi pubmed - Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, Denkert C, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-821.
doi pubmed - Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571-1580.
doi pubmed pmc - Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301.
doi pubmed - Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, Lee KH, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2021;32(9):1137-1147.
doi pubmed - Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM, Yimer HA, et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6(9):e448-e458.
doi pubmed - West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924-937.
doi pubmed - Gidaro A, Palmieri G, Donadoni M, Mameli LA, La Cava L, Sanna G, Castro D, et al. A diagnostic of acquired hemophilia following PD1/PDL1 inhibitors in advanced melanoma: the experience of two patients and a literature review. Diagnostics (Basel). 2022;12(10):2559.
doi pubmed pmc - Kasamatsu T, Ino R, Takahashi N, Gotoh N, Minato Y, Takizawa M, Yokohama A, et al. PDCD1 and CTLA4 polymorphisms affect the susceptibility to, and clinical features of, chronic immune thrombocytopenia. Br J Haematol. 2018;180(5):705-714.
doi pubmed - Hinterleitner C, Strahle J, Malenke E, Hinterleitner M, Henning M, Seehawer M, Bilich T, et al. Platelet PD-L1 reflects collective intratumoral PD-L1 expression and predicts immunotherapy response in non-small cell lung cancer. Nat Commun. 2021;12(1):7005.
doi pubmed pmc - Kroll MH, Rojas-Hernandez C, Yee C. Hematologic complications of immune checkpoint inhibitors. Blood. 2022;139(25):3594-3604.
doi pubmed pmc - Haddad TC, Zhao S, Li M, Patel SH, Johns A, Grogan M, Lopez G, et al. Immune checkpoint inhibitor-related thrombocytopenia: incidence, risk factors and effect on survival. Cancer Immunol Immunother. 2022;71(5):1157-1165.
doi pubmed pmc - Maillet D, Corbaux P, Stelmes JJ, Dalle S, Locatelli-Sanchez M, Perier-Muzet M, Duruisseaux M, et al. Association between immune-related adverse events and long-term survival outcomes in patients treated with immune checkpoint inhibitors. Eur J Cancer. 2020;132:61-70.
doi pubmed - Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020;18(1):87.
doi pubmed pmc - Shafqat H, Gourdin T, Sion A. Immune-related adverse events are linked with improved progression-free survival in patients receiving anti-PD-1/PD-L1 therapy. Semin Oncol. 2018;45(3):156-163.
doi pubmed - Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306.
doi pubmed pmc - Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, Brambilla M, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479-485.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


